| << Chapter < Page | Chapter >> Page > |
Below are some examples:
An amphoteric substance is one that can react as either an acid or base. Examples of amphoteric substances include water, zinc oxide and beryllium hydroxide.
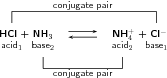
Look at the reaction between hydrochloric acid and ammonia to form ammonium and chloride ions:
Looking firstly at the forward reaction (i.e. the reaction that proceeds from left to right ), the changes that take place can be shown as follows:
and
Looking at the reverse reaction (i.e. the reaction that proceeds from right to left ), the changes that take place are as follows:
and
In the forward reaction , HCl is a proton donor (acid) and NH is a proton acceptor (base). In the reverse reaction , the chloride ion is the proton acceptor (base) and NH is the proton donor (acid). A conjugate acid-base pair is two compounds in a reaction that change into each other through the loss or gain of a proton. The conjugate acid-base pairs for the above reaction are shown below.
The reaction between ammonia and water can also be used as an example:
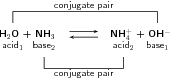
The term refers to two compounds that transform into each other by the gain or loss of a proton.
Khan academy video on acids and bases
When an acid and a base react, they neutralise each other to form a salt . If the base contains hydroxide (OH ) ions, then water will also be formed. The word salt is a general term which applies to the products of all acid-base reactions. A salt is a product that is made up of the cation from a base and the anion from an acid. When an acid reacts with a base, they neutralise each other. In other words, the acid becomes less acidic and the base becomes less basic. Look at the following examples:

Notification Switch
Would you like to follow the 'Siyavula textbooks: grade 11 physical science' conversation and receive update notifications?