| << Chapter < Page | Chapter >> Page > |
Match the information in column A with the information in column B by writing only the letter (A to I) next to the question number (1 to 7)
| 1. A positive ion that has 3 less electrons than its neutral atom | A. |
| 2. An ion that has 1 more electron than its neutral atom | B. |
| 3. The anion that is formed when bromine gains an electron | C. |
| 4. The cation that is formed from a magnesium atom | D. |
| 5. An example of a compound ion | E. |
| 6. A positive ion with the electron configuration of argon | F. |
| 7. A negative ion with the electron configuration of neon | G. |
| H. | |
| I. |
Ionisation energy is the energy that is needed to remove one electron from an atom in the gas phase. The ionisation energy will be different for different atoms.
The second ionisation energy is the energy that is needed to remove a second electron from an atom, and so on. As an energy level becomes more full, it becomes more and more difficult to remove an electron and the ionisation energy increases . On the Periodic Table of the Elements, a group is a vertical column of the elements, and a period is a horizontal row. In the periodic table, ionisation energy increases across a period, but decreases as you move down a group. The lower the ionisation energy, the more reactive the element will be because there is a greater chance of electrons being involved in chemical reactions. We will look at this in more detail in the next section.
Refer to the data table below which gives the ionisation energy (in ) and atomic number (Z) for a number of elements in the periodic table:
| Z | Ionisation energy | Z | Ionisation energy |
| 1 | 1310 | 10 | 2072 |
| 2 | 2360 | 11 | 494 |
| 3 | 517 | 12 | 734 |
| 4 | 895 | 13 | 575 |
| 5 | 797 | 14 | 783 |
| 6 | 1087 | 15 | 1051 |
| 7 | 1397 | 16 | 994 |
| 8 | 1307 | 17 | 1250 |
| 9 | 1673 | 18 | 1540 |
Khan academy video on periodic table - 2
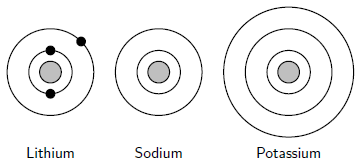
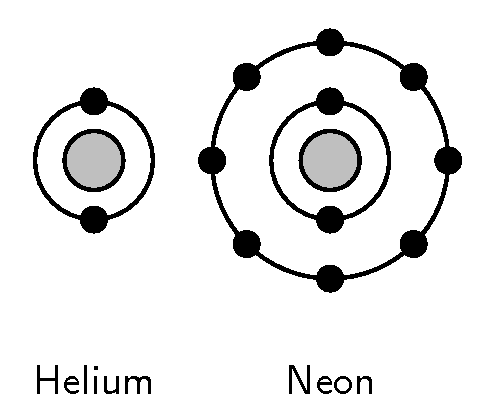
The characteristics of each group are mostly determined by the electron configuration of the atoms of the element.

Notification Switch
Would you like to follow the 'Siyavula textbooks: grade 10 physical science [caps]' conversation and receive update notifications?