| << Chapter < Page | Chapter >> Page > |
The strength of a covalent bond is measured by its bond dissociation energy, that is, the amount of energy required to break that particular bond in a mole of molecules. Multiple bonds are stronger than single bonds between the same atoms. The enthalpy of a reaction can be estimated based on the energy input required to break bonds and the energy released when new bonds are formed. For ionic bonds, the lattice energy is the energy required to separate one mole of a compound into its gas phase ions. Lattice energy increases for ions with higher charges and shorter distances between ions. Lattice energies are often calculated using the Born-Haber cycle, a thermochemical cycle including all of the energetic steps involved in converting elements into an ionic compound.
Which bond in each of the following pairs of bonds is the strongest?
(a) C–C or
(b) C–N or
(c) or
(d) H–F or H–Cl
(e) C–H or O–H
(f) C–N or C–O
Using the bond energies in [link] , determine the approximate enthalpy change for each of the following reactions:
(a)
(b)
(c)
(a) −114 kJ;
(b) 30 kJ;
(c) −1055 kJ
Using the bond energies in [link] , determine the approximate enthalpy change for each of the following reactions:
(a)
(b)
(c)
When a molecule can form two different structures, the structure with the stronger bonds is usually the more stable form. Use bond energies to predict the correct structure of the hydroxylamine molecule:
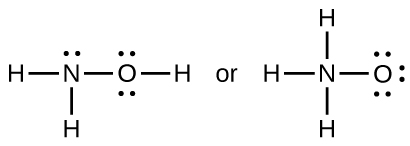
The greater bond energy is in the figure on the left. It is the more stable form.
How does the bond energy of HCl( g ) differ from the standard enthalpy of formation of HCl( g )?
Using the standard enthalpy of formation data in Appendix G , show how the standard enthalpy of formation of HCl( g ) can be used to determine the bond energy.
Using the standard enthalpy of formation data in Appendix G , calculate the bond energy of the carbon-sulfur double bond in CS 2 .
Using the standard enthalpy of formation data in Appendix G , determine which bond is stronger: the S–F bond in SF 4 ( g ) or in SF 6 ( g )?
The S–F bond in SF 4 is stronger.
Using the standard enthalpy of formation data in Appendix G , determine which bond is stronger: the P–Cl bond in PCl 3 ( g ) or in PCl 5 ( g )?
Complete the following Lewis structure by adding bonds (not atoms), and then indicate the longest bond:

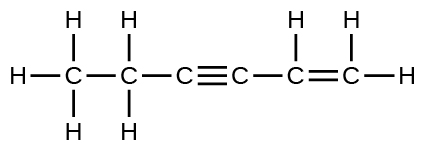
The C–C single bonds are longest.
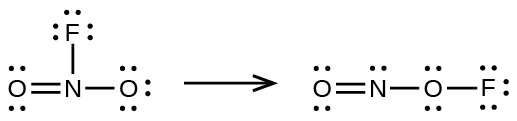
Use the bond energy to calculate an approximate value of Δ H for the following reaction. Which is the more stable form of FNO 2 ?
Use principles of atomic structure to answer each of the following: This question is taken from the Chemistry Advanced Placement Examination and is used with the permission of the Educational Testing Service.
(a) The radius of the Ca atom is 197 pm; the radius of the Ca 2+ ion is 99 pm. Account for the difference.
(b) The lattice energy of CaO( s ) is –3460 kJ/mol; the lattice energy of K 2 O is –2240 kJ/mol. Account for the difference.
(c) Given these ionization values, explain the difference between Ca and K with regard to their first and second ionization energies.
| Element | First Ionization Energy (kJ/mol) | Second Ionization Energy (kJ/mol) |
|---|---|---|
| K | 419 | 3050 |
| Ca | 590 | 1140 |
(d) The first ionization energy of Mg is 738 kJ/mol and that of Al is 578 kJ/mol. Account for this difference.
(a) When two electrons are removed from the valence shell, the Ca radius loses the outermost energy level and reverts to the lower n = 3 level, which is much smaller in radius. (b) The +2 charge on calcium pulls the oxygen much closer compared with K, thereby increasing the lattice energy relative to a less charged ion. (c) Removal of the 4 s electron in Ca requires more energy than removal of the 4 s electron in K because of the stronger attraction of the nucleus and the extra energy required to break the pairing of the electrons. The second ionization energy for K requires that an electron be removed from a lower energy level, where the attraction is much stronger from the nucleus for the electron. In addition, energy is required to unpair two electrons in a full orbital. For Ca, the second ionization potential requires removing only a lone electron in the exposed outer energy level. (d) In Al, the removed electron is relatively unprotected and unpaired in a p orbital. The higher energy for Mg mainly reflects the unpairing of the 2 s electron.
The lattice energy of LiF is 1023 kJ/mol, and the Li–F distance is 200.8 pm. NaF crystallizes in the same structure as LiF but with a Na–F distance of 231 pm. Which of the following values most closely approximates the lattice energy of NaF: 510, 890, 1023, 1175, or 4090 kJ/mol? Explain your choice.
For which of the following substances is the least energy required to convert one mole of the solid into separate ions?
(a) MgO
(b) SrO
(c) KF
(d) CsF
(e) MgF 2
(d)
The reaction of a metal, M, with a halogen, X 2 , proceeds by an exothermic reaction as indicated by this equation: For each of the following, indicate which option will make the reaction more exothermic. Explain your answers.
(a) a large radius vs. a small radius for M +2
(b) a high ionization energy vs. a low ionization energy for M
(c) an increasing bond energy for the halogen
(d) a decreasing electron affinity for the halogen
(e) an increasing size of the anion formed by the halogen
The lattice energy of LiF is 1023 kJ/mol, and the Li–F distance is 201 pm. MgO crystallizes in the same structure as LiF but with a Mg–O distance of 205 pm. Which of the following values most closely approximates the lattice energy of MgO: 256 kJ/mol, 512 kJ/mol, 1023 kJ/mol, 2046 kJ/mol, or 4008 kJ/mol? Explain your choice.
4008 kJ/mol; both ions in MgO have twice the charge of the ions in LiF; the bond length is very similar and both have the same structure; a quadrupling of the energy is expected based on the equation for lattice energy
Which compound in each of the following pairs has the larger lattice energy? Note: Mg 2+ and Li + have similar radii; O 2– and F – have similar radii. Explain your choices.
(a) MgO or MgSe
(b) LiF or MgO
(c) Li 2 O or LiCl
(d) Li 2 Se or MgO
Which compound in each of the following pairs has the larger lattice energy? Note: Ba 2+ and
K + have similar radii; S 2– and Cl – have similar radii. Explain your choices.
(a) K 2 O or Na 2 O
(b) K 2 S or BaS
(c) KCl or BaS
(d) BaS or BaCl 2
(a) Na 2 O; Na + has a smaller radius than K + ; (b) BaS; Ba has a larger charge than K; (c) BaS; Ba and S have larger charges; (d) BaS; S has a larger charge
Which of the following compounds requires the most energy to convert one mole of the solid into separate ions?
(a) MgO
(b) SrO
(c) KF
(d) CsF
(e) MgF 2
Which of the following compounds requires the most energy to convert one mole of the solid into separate ions?
(a) K 2 S
(b) K 2 O
(c) CaS
(d) Cs 2 S
(e) CaO
(e)
The lattice energy of KF is 794 kJ/mol, and the interionic distance is 269 pm. The Na–F
distance in NaF, which has the same structure as KF, is 231 pm. Which of the following values is the closest approximation of the lattice energy of NaF: 682 kJ/mol, 794 kJ/mol, 924 kJ/mol, 1588 kJ/mol, or 3175 kJ/mol? Explain your answer.

Notification Switch
Would you like to follow the 'Chemistry' conversation and receive update notifications?