| << Chapter < Page | Chapter >> Page > |
Thus, the mass of the rocket is
This result means that only of the mass is left when the fuel is burnt, and of the initial mass was fuel. Expressed as percentages, 98.9% of the rocket is fuel, while payload, engines, fuel tanks, and other components make up only 1.10%. Taking air resistance and gravitational force into account, the mass remaining can only be about . It is difficult to build a rocket in which the fuel has a mass 180 times everything else. The solution is multistage rockets. Each stage only needs to achieve part of the final velocity and is discarded after it burns its fuel. The result is that each successive stage can have smaller engines and more payload relative to its fuel. Once out of the atmosphere, the ratio of payload to fuel becomes more favorable, too.
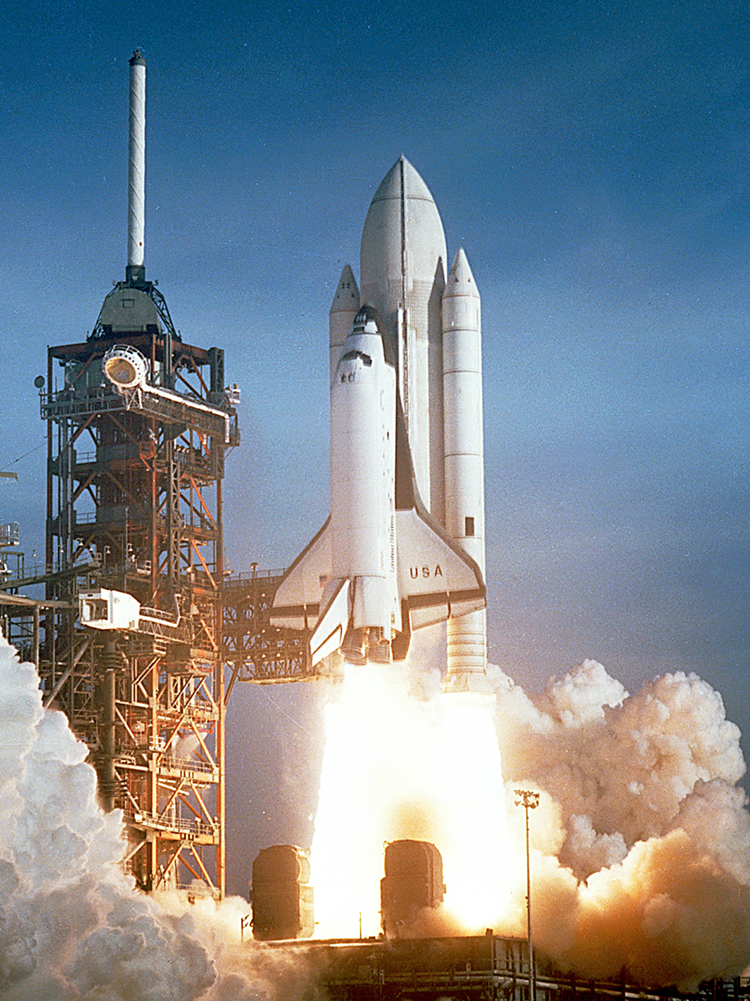
The space shuttle was an attempt at an economical vehicle with some reusable parts, such as the solid fuel boosters and the craft itself. (See [link] ) The shuttle’s need to be operated by humans, however, made it at least as costly for launching satellites as expendable, unmanned rockets. Ideally, the shuttle would only have been used when human activities were required for the success of a mission, such as the repair of the Hubble space telescope. Rockets with satellites can also be launched from airplanes. Using airplanes has the double advantage that the initial velocity is significantly above zero and a rocket can avoid most of the atmosphere’s resistance.
Can you avoid the boulder field and land safely, just before your fuel runs out, as Neil Armstrong did in 1969? Our version of this classic video game accurately simulates the real motion of the lunar lander with the correct mass, thrust, fuel consumption rate, and lunar gravity. The real lunar lander is very hard to control.

Professional Application
Suppose a fireworks shell explodes, breaking into three large pieces for which air resistance is negligible. How is the motion of the center of mass affected by the explosion? How would it be affected if the pieces experienced significantly more air resistance than the intact shell?

Notification Switch
Would you like to follow the 'College physics' conversation and receive update notifications?