| << Chapter < Page | Chapter >> Page > |
As the example implies, gravitational force is completely negligible on a small scale, where the interactions of individual charged particles are important. On a large scale, such as between the Earth and a person, the reverse is true. Most objects are nearly electrically neutral, and so attractive and repulsive Coulomb forces nearly cancel. Gravitational force on a large scale dominates interactions between large objects because it is always attractive, while Coulomb forces tend to cancel.
where and are two point charges separated by a distance , and
[link] shows the charge distribution in a water molecule, which is called a polar molecule because it has an inherent separation of charge. Given water’s polar character, explain what effect humidity has on removing excess charge from objects.
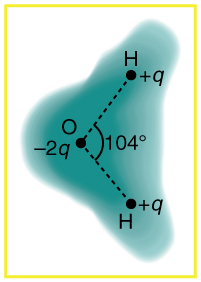
Using [link] , explain, in terms of Coulomb’s law, why a polar molecule (such as in [link] ) is attracted by both positive and negative charges.
Given the polar character of water molecules, explain how ions in the air form nucleation centers for rain droplets.
What is the repulsive force between two pith balls that are 8.00 cm apart and have equal charges of – 30.0 nC?
(a) How strong is the attractive force between a glass rod with a charge and a silk cloth with a charge, which are 12.0 cm apart, using the approximation that they act like point charges? (b) Discuss how the answer to this problem might be affected if the charges are distributed over some area and do not act like point charges.
(a) 0.263 N
(b) If the charges are distributed over some area, there will be a concentration of charge along the side closest to the oppositely charged object. This effect will increase the net force.
Two point charges exert a 5.00 N force on each other. What will the force become if the distance between them is increased by a factor of three?
Two point charges are brought closer together, increasing the force between them by a factor of 25. By what factor was their separation decreased?
The separation decreased by a factor of 5.

Notification Switch
Would you like to follow the 'College physics' conversation and receive update notifications?