| << Chapter < Page | Chapter >> Page > |
It is also possible to change the temperature of a substance by doing work. Work can transfer energy into or out of a system. This realization helped establish the fact that heat is a form of energy. James Prescott Joule (1818–1889) performed many experiments to establish the mechanical equivalent of heat — the work needed to produce the same effects as heat transfer . In terms of the units used for these two terms, the best modern value for this equivalence is
We consider this equation as the conversion between two different units of energy.
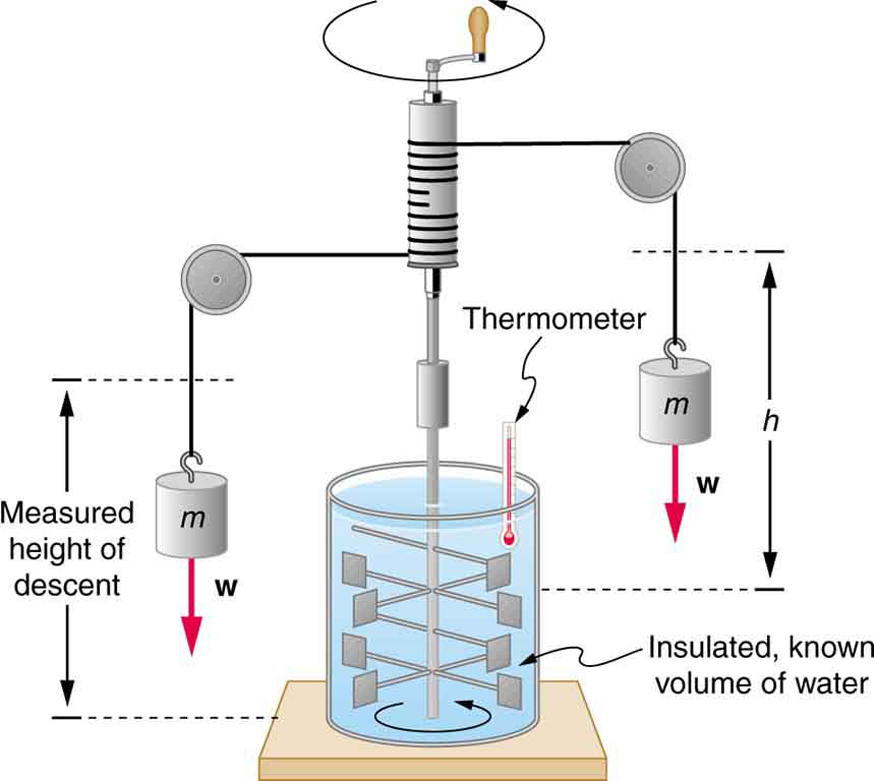
The figure above shows one of Joule’s most famous experimental setups for demonstrating the mechanical equivalent of heat. It demonstrated that work and heat can produce the same effects, and helped establish the principle of conservation of energy. Gravitational potential energy (PE) (work done by the gravitational force) is converted into kinetic energy (KE), and then randomized by viscosity and turbulence into increased average kinetic energy of atoms and molecules in the system, producing a temperature increase. His contributions to the field of thermodynamics were so significant that the SI unit of energy was named after him.
Heat added or removed from a system changes its internal energy and thus its temperature. Such a temperature increase is observed while cooking. However, adding heat does not necessarily increase the temperature. An example is melting of ice; that is, when a substance changes from one phase to another. Work done on the system or by the system can also change the internal energy of the system. Joule demonstrated that the temperature of a system can be increased by stirring. If an ice cube is rubbed against a rough surface, work is done by the frictional force. A system has a well-defined internal energy, but we cannot say that it has a certain “heat content” or “work content”. We use the phrase “heat transfer” to emphasize its nature.
Two samples (A and B) of the same substance are kept in a lab. Someone adds 10 kilojoules (kJ) of heat to one sample, while 10 kJ of work is done on the other sample. How can you tell to which sample the heat was added?
Heat and work both change the internal energy of the substance. However, the properties of the sample only depend on the internal energy so that it is impossible to tell whether heat was added to sample A or B.
An ice cube is placed in a cup of hot water. Which of the following statements correctly describes energy transfer at the molecular level?
(c)
The molecular description of heat transfer from higher to lower temperatures applies to ‘spontaneous’ processes, that is, processes in which no energy is added to or removed or from the systems by work or heat. Refrigeration is an example of work being done to remove energy from air within a given space, and thus lower the temperature of the air. Assume a typical kitchen refrigerator, where the air inside the unit forms the system with a temperature of 25°C, and the walls are kept at a constant temperature of 10°C. In terms of molecules and average kinetic energy, describe how the air is made colder.
How is heat transfer related to temperature?
Describe a situation in which heat transfer occurs. What are the resulting forms of energy?
When heat transfers into a system, is the energy stored as heat? Explain briefly.

Notification Switch
Would you like to follow the 'College physics for ap® courses' conversation and receive update notifications?