| << Chapter < Page | Chapter >> Page > |
On the submicroscopic scale, it is more convenient to define an energy unit called the electron volt (eV), which is the energy given to a fundamental charge accelerated through a potential difference of 1 V. In equation form,
On the submicroscopic scale, it is more convenient to define an energy unit called the electron volt (eV), which is the energy given to a fundamental charge accelerated through a potential difference of 1 V . In equation form,
An electron accelerated through a potential difference of 1 V is given an energy of 1 eV. It follows that an electron accelerated through 50 V is given 50 eV. A potential difference of 100,000 V (100 kV) will give an electron an energy of 100,000 eV (100 keV), and so on. Similarly, an ion with a double positive charge accelerated through 100 V will be given 200 eV of energy. These simple relationships between accelerating voltage and particle charges make the electron volt a simple and convenient energy unit in such circumstances.
The electron volt (eV) is the most common energy unit for submicroscopic processes. This will be particularly noticeable in the chapters on modern physics. Energy is so important to so many subjects that there is a tendency to define a special energy unit for each major topic. There are, for example, calories for food energy, kilowatt-hours for electrical energy, and therms for natural gas energy.
The electron volt is commonly employed in submicroscopic processes—chemical valence energies and molecular and nuclear binding energies are among the quantities often expressed in electron volts. For example, about 5 eV of energy is required to break up certain organic molecules. If a proton is accelerated from rest through a potential difference of 30 kV, it is given an energy of 30 keV (30,000 eV) and it can break up as many as 6000 of these molecules ( ). Nuclear decay energies are on the order of 1 MeV (1,000,000 eV) per event and can, thus, produce significant biological damage.
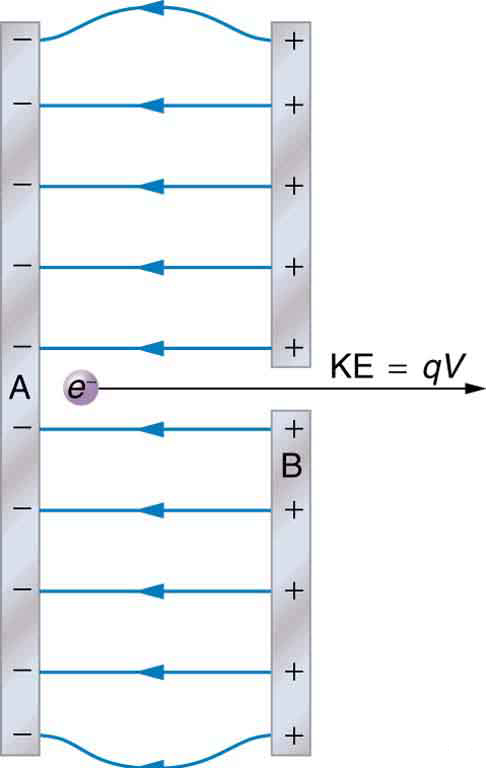
The total energy of a system is conserved if there is no net addition (or subtraction) of work or heat transfer. For conservative forces, such as the electrostatic force, conservation of energy states that mechanical energy is a constant.
Mechanical energy is the sum of the kinetic energy and potential energy of a system; that is, . A loss of PE of a charged particle becomes an increase in its KE. Here PE is the electric potential energy. Conservation of energy is stated in equation form as
or
where i and f stand for initial and final conditions. As we have found many times before, considering energy can give us insights and facilitate problem solving.

Notification Switch
Would you like to follow the 'General physics ii phy2202ca' conversation and receive update notifications?