| << Chapter < Page | Chapter >> Page > |
We already know that removing an electron from an atom requires energy, so it must be true that removing an electron from a metal requires energy, as well. In the photoelectric effect, the energy required is provided by the light source. We can vary the frequency of the light source and the intensity of the light source to determine under what conditions enough energy is provided to eject an electron from the metal. This could be detected by measuring the electric current produced. The more electrons ejected, the greater the current. We can also measure the energy of the ejected electrons. This is hard to do, but it is possible to measure the kinetic energy of these electrons as they are collected.
Before looking at the experimental data, we can stop to make a prediction about the results. We know that more intense light must contain greater energy, since light from the sun can certainly warm something up faster than a flashlight. So we would expect that more intense light would produce more and faster electrons. What would be harder to predict is the effect of changing the frequency of the light. So first, let’s use a light source with a single frequency. This can be done by passing the light source first through a prism or diffraction grating. Then we can vary the intensity of the light.
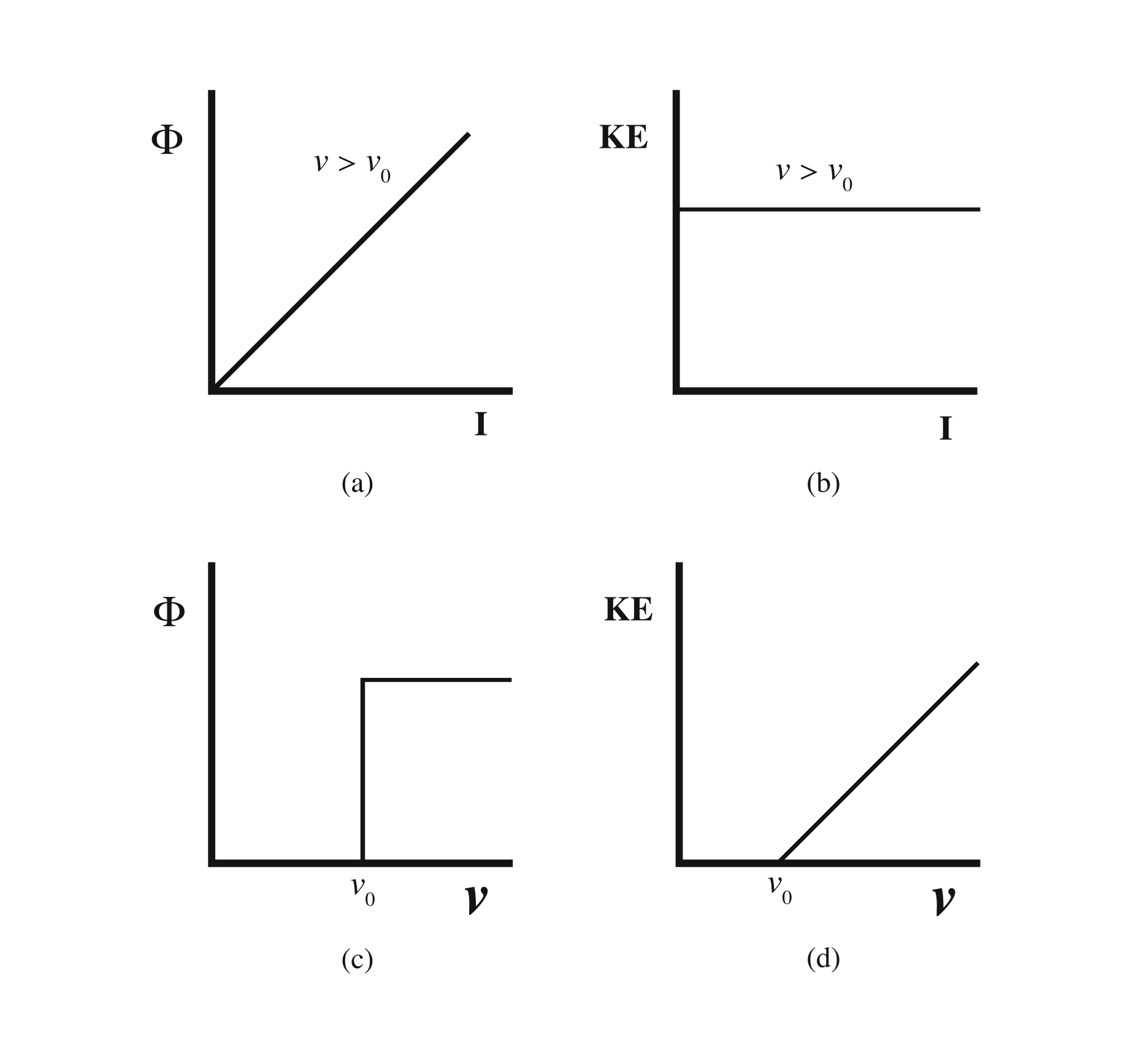
The first surprising result is that, if we choose a frequency too low for our source, there are no electrons produced by the photoelectric effect. There is no current at all. We have to use a light source with at least a minimum frequency, ν 0 , called the “threshold frequency,” in order to observe electrons ejected. This is puzzling. Even when we apply a very intense light source, which presumably provides a lot of energy, no electrons are ejected unless we are using light of at least the minimum frequency. And conversely, even if the intensity is very low so that not much energy is provided, we do get electrons ejected if the light source has frequency above the threshold. This is not what we would expect.
The value of the threshold frequency depends on which type of metal we shine the light source on. Let’s stick with a single type of metal, and let’s use a light source with frequency greater than the threshold, so ν > ν 0 . With this light source in place, we’ll vary the intensity of the light and measure the current and the kinetic energy of the ejected electrons. The results are shown in [link] .

Notification Switch
Would you like to follow the 'Concept development studies in chemistry 2013' conversation and receive update notifications?