| << Chapter < Page | Chapter >> Page > |
The temperature of the troposphere is warm (roughly 17º C) near the surface of the earth. This is due to the absorption of infrared radiation from the surface by water vapor and other greenhouse gases (e.g. carbon dioxide, nitrous oxide and methane) in the troposphere. The concentration of these gases decreases with altitude, and therefore, the heating effect is greatest near the surface. The temperature in the troposphere decreases at a rate of roughly 6.5º C per kilometer of altitude. The temperature at its upper boundary is very cold (roughly -60º C).
Because hot air rises and cold air falls, there is a constant convective overturn of material in the troposphere. Indeed, the name troposphere means “region of mixing.” For this reason, all weather phenomena occur in the troposphere. Water vapor evaporated from the earth's surface condenses in the cooler upper regions of the troposphere and falls back to the surface as rain. Dust and pollutants injected into the troposphere become well mixed in the layer, but are eventually washed out by rainfall. The troposphere is therefore self cleaning.
A narrow zone at the top of the troposphere is called the tropopause . It effectively separates the underlying troposphere and the overlying stratosphere. The temperature in the tropopause is relatively constant. Strong eastward winds, known as the jet stream , also occur here.
The stratosphere is the next major atmospheric layer. This layer extends from the tropopause (roughly 12 kilometers) to roughly 50 kilometers above the earth's surface. The temperature profile of the stratosphere is quite different from that of the troposphere. The temperature remains relatively constant up to roughly 25 kilometers and then gradually increases up to the upper boundary of the layer. The amount of water vapor in the stratosphere is very low, so it is not an important factor in the temperature regulation of the layer. Instead, it is ozone (O3) that causes the observed temperature inversion.
Most of the ozone in the atmosphere is contained in a layer of the stratosphere from roughly 20 to 30 kilometers. This ozone layer absorbs solar energy in the form of ultraviolet radiation (UV), and the energy is ultimately dissipated as heat in the stratosphere. This heat leads to the rise in temperature. Stratospheric ozone is also very important for living organisms on the surface of the earth as it protects them by absorbing most of the harmful UV radiation from the sun. Ozone is constantly being produced and destroyed in the stratosphere in a natural cycle. The basic reactions involving only oxygen (known as the " Chapman Reactions ") are as follows:
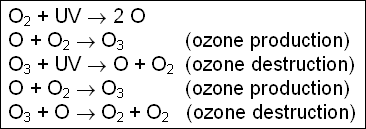
The production of ozone from molecular oxygen involves the absorption of high energy UV radiation (UVA) in the upper atmosphere. The destruction of ozone by absorption of UV radiation involves moderate and low energy radiation (UVB and UVC). Most of the production and destruction of ozone occurs in the stratosphere at lower latitudes where the ultraviolet radiation is most intense.

Notification Switch
Would you like to follow the 'Ap environmental science' conversation and receive update notifications?