| << Chapter < Page | Chapter >> Page > |
One immediate consequence of this observation is that it provides us an “absolute temperature scale” with a zero that is not arbitrarily defined. Since
V = α (t + β/α)
then the gas volume is proportional to t + β/α where t is in °C. This means it would be useful to define a temperature scale T = t + β/α = t + 273. This new scale is called the absolute temperature scale and the units are in Kelvin (K). Note that the size of the unit K is the same as the size of the unit °C; for example, the temperature change of 1 K between 373 K and 374 K is the same as the temperature change of 1 °C between 100 °C and 101 °C.
In this new temperature scale, V = αT. This means that the volume is proportional to the absolute temperature in Kelvin, provided that the pressure and amount of gas are held constant. This result is known as Charles’ Law, dating to 1787.
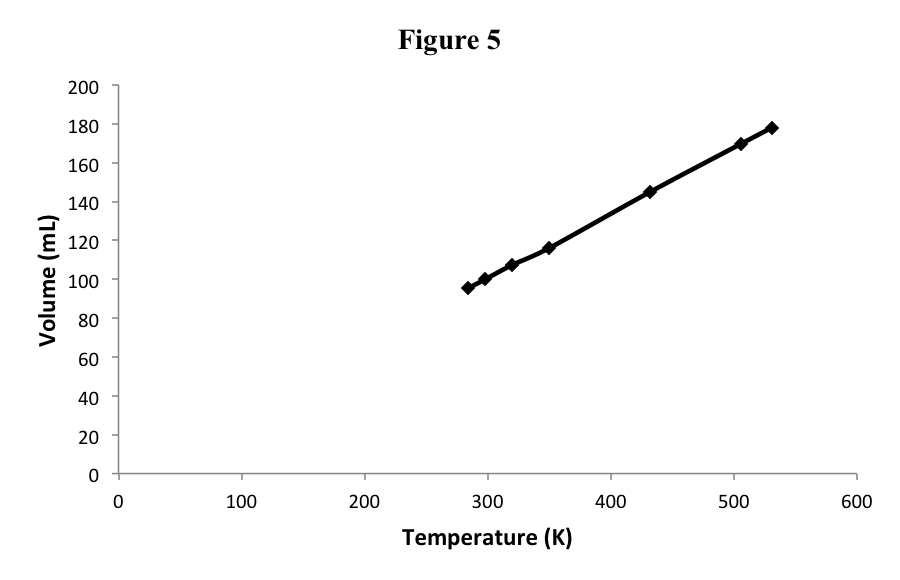
We can see this result in the data in Table 4, which are now recalibrated to the absolute temperature scale, and in Figure 5.
Table 4
| Temperature (°C) | Temperature (K) | Volume (mL) |
| 11 | 284 | 95.3 |
| 25 | 298 | 100.0 |
| 47 | 320 | 107.4 |
| 73 | 350 | 116.1 |
| 159 | 432 | 145.0 |
| 233 | 506 | 169.8 |
| 258 | 531 | 178.1 |
As with Boyle’s Law, we must now note that the “constant” α is not really constant, since the volume also depends on the pressure and quantity of gas. Also as with Boyle’s Law, we note that Charles’ Law does not depend on the type of gas on which we make the measurements, but rather it depends only the number of particles of gas. Therefore, we slightly rewrite Charles’ Law to explicitly indicate the dependence of α on the pressure and number of particles of gas:
V = α(N,P) T
We have been measuring four properties of gases: pressure, volume, temperature, and “amount,” which we have assumed above to be the number of particles through Avogadro’s Law. The results of three observations relate these four properties pairwise. Boyle’s Law relates the pressure and volume at constant temperature and amount of gas:
P × V = k B (N,T)
where we have given the Boyle’s Law function the name k B (B is for Boyle) to distinguish it from other k’s we might encounter.
Charles’ Law relates the volume and temperature at constant pressure and amount of gas:
V = k C (N,P) T
where we have given the Charles’s Law function the name k C (C is for Charles) to distinguish it.
Finally, the Law of Combining Volumes leads to Avogadro’s Law that the volume of a gas is proportional to the number of particles (N) provided that the temperature and pressure are held constant. If we let k A (A is for Avogadro) be the proportionality constant between V and N, then C is a function of P and T. We can express this as
V = k A (P,T) N
We will demonstrate below that these three relationships can be combined into a single equation relating P, V, T, and N. Jumping to the conclusion, however, we can more easily show that these three relationships can be considered as special cases of the more general equation known as the Ideal Gas Law:
PV = nRT
where R is a constant, n is the number of moles of gas, related to the number of particles N by Avogadro’s number, N A :

Notification Switch
Would you like to follow the 'Concept development studies in chemistry 2013' conversation and receive update notifications?