| << Chapter < Page | Chapter >> Page > |
If we partially fill an airtight syringe with air, the syringe contains a specific amount of air at constant temperature, say 25 °C. If we slowly push in the plunger while keeping temperature constant, the gas in the syringe is compressed into a smaller volume and its pressure increases; if we pull out the plunger, the volume increases and the pressure decreases. This example of the effect of volume on the pressure of a given amount of a confined gas is true in general. Decreasing the volume of a contained gas will increase its pressure, and increasing its volume will decrease its pressure. In fact, if the volume increases by a certain factor, the pressure decreases by the same factor, and vice versa. Volume-pressure data for an air sample at room temperature are graphed in [link] .
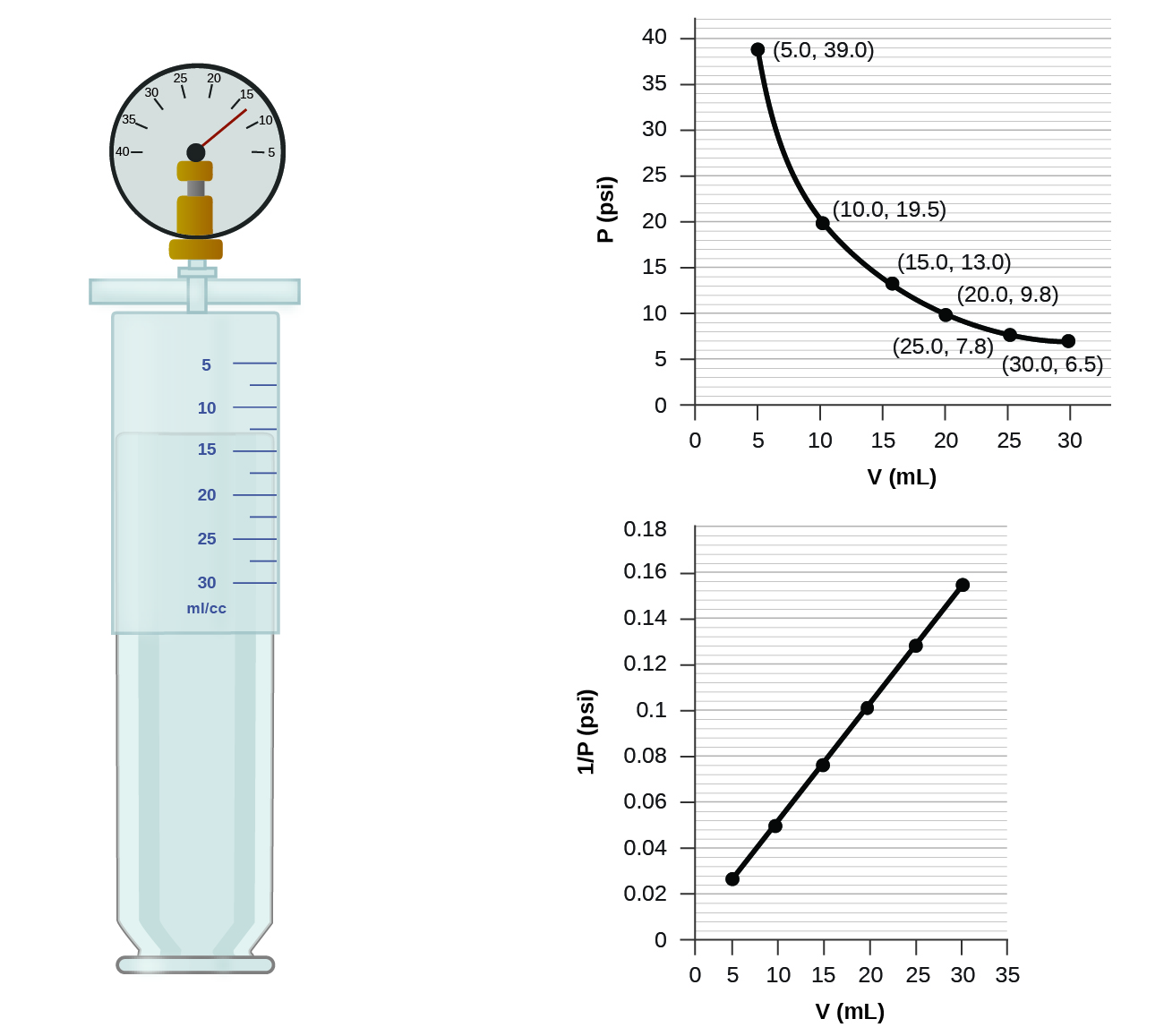
Unlike the P - T and V - T relationships, pressure and volume are not directly proportional to each other. Instead, P and V exhibit inverse proportionality: Increasing the pressure results in a decrease of the volume of the gas. Mathematically this can be written:
with k being a constant. Graphically, this relationship is shown by the straight line that results when plotting the inverse of the pressure versus the volume ( V ), or the inverse of volume versus the pressure ( P ). Graphs with curved lines are difficult to read accurately at low or high values of the variables, and they are more difficult to use in fitting theoretical equations and parameters to experimental data. For those reasons, scientists often try to find a way to “linearize” their data. If we plot P versus V , we obtain a hyperbola (see [link] ).
The relationship between the volume and pressure of a given amount of gas at constant temperature was first published by the English natural philosopher Robert Boyle over 300 years ago. It is summarized in the statement now known as Boyle’s law : The volume of a given amount of gas held at constant temperature is inversely proportional to the pressure under which it is measured.
(a) the P - V graph in [link]
(b) the vs. V graph in [link]
(c) the Boyle’s law equation
Comment on the likely accuracy of each method.
(b) Estimating from the versus V graph give a value of about 26 psi.
(c) From Boyle’s law, we know that the product of pressure and volume ( PV ) for a given sample of gas at a constant temperature is always equal to the same value. Therefore we have P 1 V 1 = k and P 2 V 2 = k which means that P 1 V 1 = P 2 V 2 .
Using P 1 and V 1 as the known values 13.0 psi and 15.0 mL, P 2 as the pressure at which the volume is unknown, and V 2 as the unknown volume, we have:
Solving:
It was more difficult to estimate well from the P - V graph, so (a) is likely more inaccurate than (b) or (c). The calculation will be as accurate as the equation and measurements allow.
(a) the P - V graph in [link]
(b) the vs. V graph in [link]
(c) the Boyle’s law equation
Comment on the likely accuracy of each method.
(a) about 17–18 mL; (b) ~18 mL; (c) 17.7 mL; it was more difficult to estimate well from the P - V graph, so (a) is likely more inaccurate than (b); the calculation will be as accurate as the equation and measurements allow

Notification Switch
Would you like to follow the 'Chemistry' conversation and receive update notifications?