| << Chapter < Page | Chapter >> Page > |
When atomic orbitals hybridize, the valence electrons occupy the newly created orbitals. The Be atom had two valence electrons, so each of the sp orbitals gets one of these electrons. Each of these electrons pairs up with the unpaired electron on a chlorine atom when a hybrid orbital and a chlorine orbital overlap during the formation of the Be–Cl bonds.
Any central atom surrounded by just two regions of valence electron density in a molecule will exhibit sp hybridization. Other examples include the mercury atom in the linear HgCl 2 molecule, the zinc atom in Zn(CH 3 ) 2 , which contains a linear C–Zn–C arrangement, and the carbon atoms in HCCH and CO 2 .
Check out the University of Wisconsin-Oshkosh website to learn about visualizing hybrid orbitals in three dimensions.
The valence orbitals of a central atom surrounded by three regions of electron density consist of a set of three sp 2 hybrid orbitals and one unhybridized p orbital. This arrangement results from sp 2 hybridization, the mixing of one s orbital and two p orbitals to produce three identical hybrid orbitals oriented in a trigonal planar geometry ( [link] ).
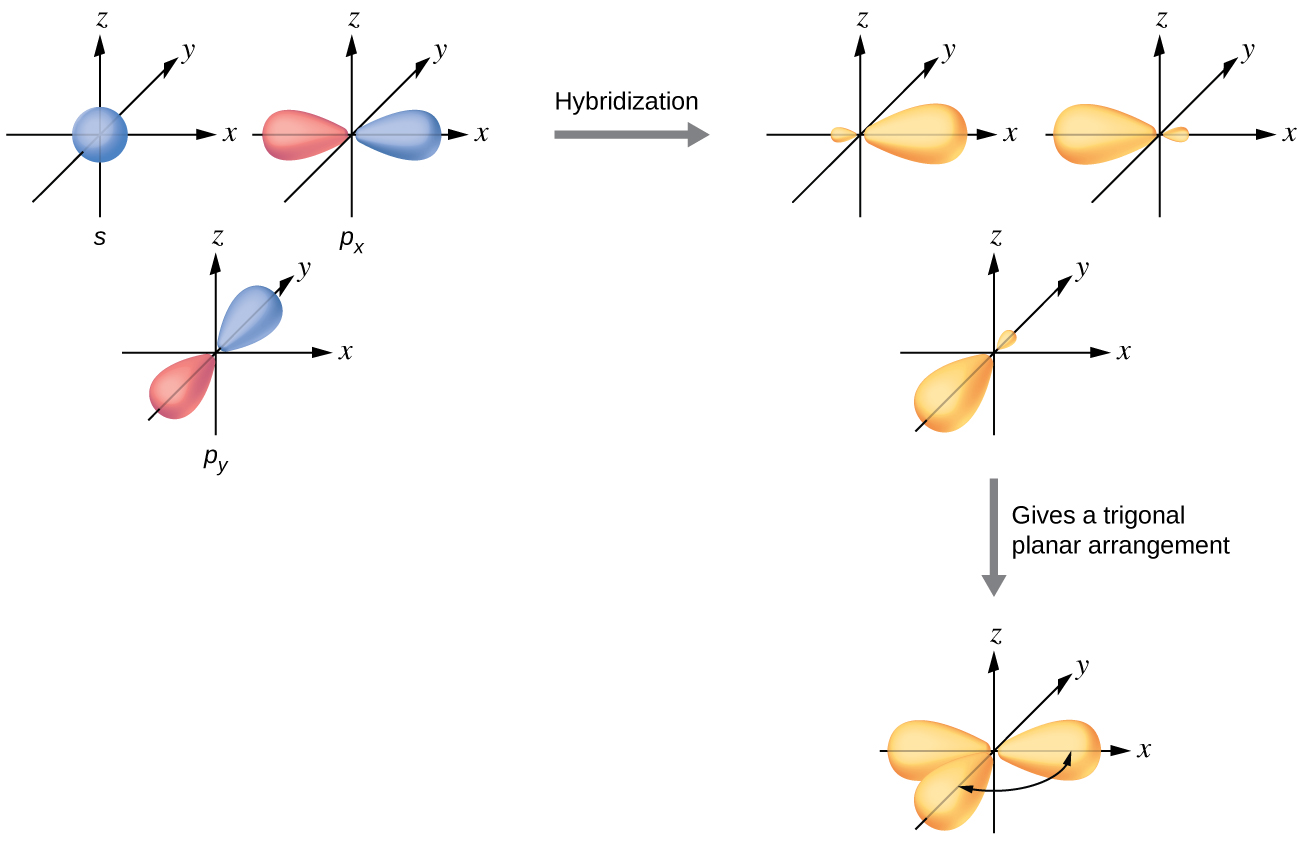
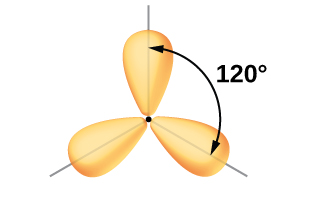
Although quantum mechanics yields the “plump” orbital lobes as depicted in [link] , sometimes for clarity these orbitals are drawn thinner and without the minor lobes, as in [link] , to avoid obscuring other features of a given illustration. We will use these “thinner” representations whenever the true view is too crowded to easily visualize.
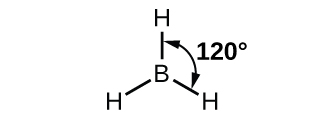
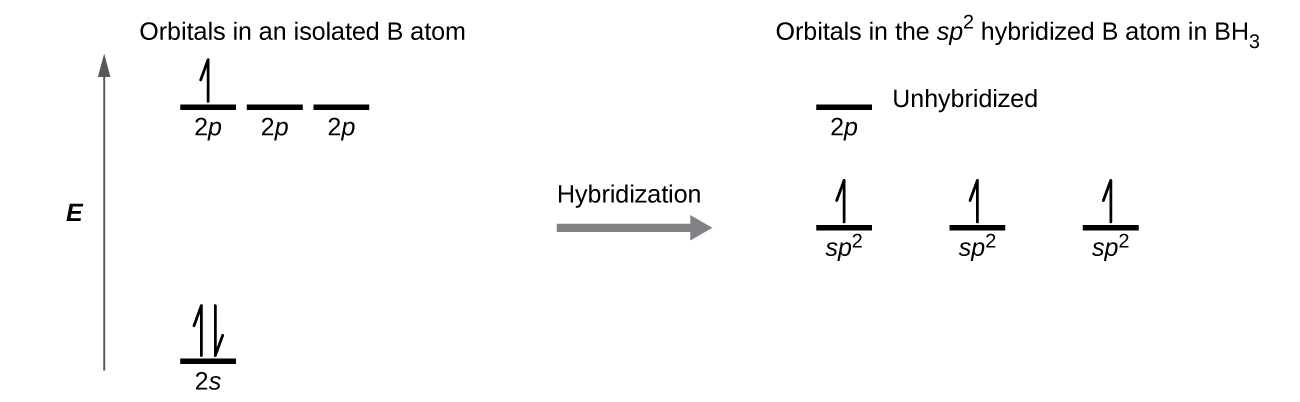
The observed structure of the borane molecule, BH 3, suggests sp 2 hybridization for boron in this compound. The molecule is trigonal planar, and the boron atom is involved in three bonds to hydrogen atoms ( [link] ). We can illustrate the comparison of orbitals and electron distribution in an isolated boron atom and in the bonded atom in BH 3 as shown in the orbital energy level diagram in [link] . We redistribute the three valence electrons of the boron atom in the three sp 2 hybrid orbitals, and each boron electron pairs with a hydrogen electron when B–H bonds form.
Any central atom surrounded by three regions of electron density will exhibit sp 2 hybridization. This includes molecules with a lone pair on the central atom, such as ClNO ( [link] ), or molecules with two single bonds and a double bond connected to the central atom, as in formaldehyde, CH 2 O, and ethene, H 2 CCH 2 .

Notification Switch
Would you like to follow the 'Chemistry' conversation and receive update notifications?