| << Chapter < Page | Chapter >> Page > |
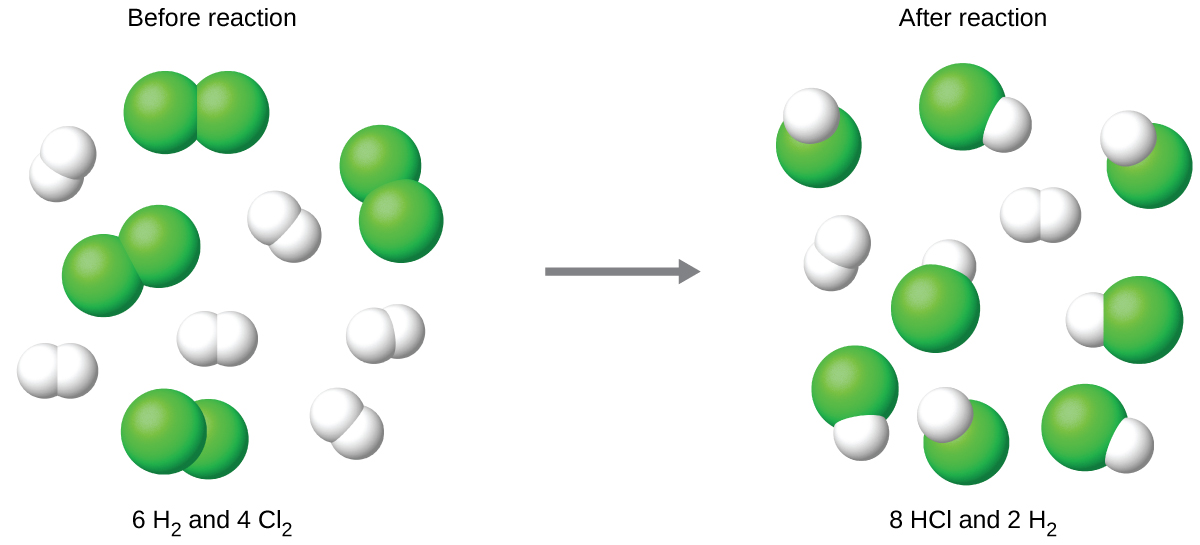
Complete reaction of the provided chlorine would produce
The chlorine will be completely consumed once 4 moles of HCl have been produced. Since enough hydrogen was provided to yield 6 moles of HCl, there will be unreacted hydrogen remaining once this reaction is complete. Chlorine, therefore, is the limiting reactant and hydrogen is the excess reactant ( [link] ).

View this interactive simulation illustrating the concepts of limiting and excess reactants.
Which is the limiting reactant when 2.00 g of Si and 1.50 g of N 2 react?
The provided Si:N 2 molar ratio is:
The stoichiometric Si:N 2 ratio is:
Comparing these ratios shows that Si is provided in a less-than-stoichiometric amount, and so is the limiting reactant.
Alternatively, compute the amount of product expected for complete reaction of each of the provided reactants. The 0.0712 moles of silicon would yield
while the 0.0535 moles of nitrogen would produce
Since silicon yields the lesser amount of product, it is the limiting reactant.
O 2
The amount of product that may be produced by a reaction under specified conditions, as calculated per the stoichiometry of an appropriate balanced chemical equation, is called the theoretical yield of the reaction. In practice, the amount of product obtained is called the actual yield , and it is often less than the theoretical yield for a number of reasons. Some reactions are inherently inefficient, being accompanied by side reactions that generate other products. Others are, by nature, incomplete (consider the partial reactions of weak acids and bases discussed earlier in this chapter). Some products are difficult to collect without some loss, and so less than perfect recovery will reduce the actual yield. The extent to which a reaction’s theoretical yield is achieved is commonly expressed as its percent yield :
Actual and theoretical yields may be expressed as masses or molar amounts (or any other appropriate property; e.g., volume, if the product is a gas). As long as both yields are expressed using the same units, these units will cancel when percent yield is calculated.

Notification Switch
Would you like to follow the 'Chemistry' conversation and receive update notifications?