| << Chapter < Page | Chapter >> Page > |
When reactions are carried out using less-than-stoichiometric quantities of reactants, the amount of product generated will be determined by the limiting reactant. The amount of product generated by a chemical reaction is its actual yield. This yield is often less than the amount of product predicted by the stoichiometry of the balanced chemical equation representing the reaction (its theoretical yield). The extent to which a reaction generates the theoretical amount of product is expressed as its percent yield.
The following quantities are placed in a container: 1.5 10 24 atoms of hydrogen, 1.0 mol of sulfur, and 88.0 g of diatomic oxygen.
(a) What is the total mass in grams for the collection of all three elements?
(b) What is the total number of moles of atoms for the three elements?
(c) If the mixture of the three elements formed a compound with molecules that contain two hydrogen atoms, one sulfur atom, and four oxygen atoms, which substance is consumed first?
(d) How many atoms of each remaining element would remain unreacted in the change described in (c)?
What is the limiting reactant in a reaction that produces sodium chloride from 8 g of sodium and 8 g of diatomic chlorine?
The limiting reactant is Cl2.
Which of the postulates of Dalton's atomic theory explains why we can calculate a theoretical yield for a chemical reaction?
A student isolated 25 g of a compound following a procedure that would theoretically yield 81 g. What was his percent yield?
A sample of 0.53 g of carbon dioxide was obtained by heating 1.31 g of calcium carbonate. What is the percent yield for this reaction?
Freon-12, CCl 2 F 2 , is prepared from CCl 4 by reaction with HF. The other product of this reaction is HCl. Outline the steps needed to determine the percent yield of a reaction that produces 12.5 g of CCl 2 F 2 from 32.9 g of CCl 4 . Freon-12 has been banned and is no longer used as a refrigerant because it catalyzes the decomposition of ozone and has a very long lifetime in the atmosphere. Determine the percent yield.
Citric acid, C
6 H
8 O
7 , a component of jams, jellies, and fruity soft drinks, is prepared industrially via fermentation of sucrose by the mold
Aspergillus niger . The equation representing this reaction is
What mass of citric acid is produced from exactly 1 metric ton (1.000 10 3 kg) of sucrose if the yield is 92.30%?
Toluene, C 6 H 5 CH 3 , is oxidized by air under carefully controlled conditions to benzoic acid, C 6 H 5 CO 2 H, which is used to prepare the food preservative sodium benzoate, C 6 H 5 CO 2 Na. What is the percent yield of a reaction that converts 1.000 kg of toluene to 1.21 kg of benzoic acid?
In a laboratory experiment, the reaction of 3.0 mol of H 2 with 2.0 mol of I 2 produced 1.0 mol of HI. Determine the theoretical yield in grams and the percent yield for this reaction.
Outline the steps needed to solve the following problem, then do the calculations. Ether, (C 2 H 5 ) 2 O, which was originally used as an anesthetic but has been replaced by safer and more effective medications, is prepared by the reaction of ethanol with sulfuric acid.
2C 2 H 5 OH + H 2 SO 4 ⟶ (C 2 H 5 ) 2 + H 2 SO 4 ·H 2 O
What is the percent yield of ether if 1.17 L (d = 0.7134 g/mL) is isolated from the reaction of 1.500 L of C
2 H
5 OH
(d = 0.7894 g/mL)?
Convert mass of ethanol to moles of ethanol; relate the moles of ethanol to the moles of ether produced using the stoichiometry of the balanced equation. Convert moles of ether to grams; divide the actual grams of ether (determined through the density) by the theoretical mass to determine the percent yield; 87.6%
Outline the steps needed to determine the limiting reactant when 30.0 g of propane, C 3 H 8 , is burned with 75.0 g of oxygen.
Determine the limiting reactant.
Outline the steps needed to determine the limiting reactant when 0.50 mol of Cr and 0.75 mol of H
3 PO
4 react according to the following chemical equation.
Determine the limiting reactant.
The conversion needed is Then compare the amount of Cr to the amount of acid present. Cr is the limiting reactant.
What is the limiting reactant when 1.50 g of lithium and 1.50 g of nitrogen combine to form lithium nitride, a component of advanced batteries, according to the following unbalanced equation?
Uranium can be isolated from its ores by dissolving it as UO 2 (NO 3 ) 2 , then separating it as solid UO 2 (C 2 O 4 )·3H 2 O. Addition of 0.4031 g of sodium oxalate, Na 2 C 2 O 4 , to a solution containing 1.481 g of uranyl nitrate, UO 2 (NO 3 ) 2 , yields 1.073 g of solid UO 2 (C 2 O 4 )·3H 2 O.
Na 2 C 2 O 4 + UO 2 (NO 3 ) 2 + 3H 2 O ⟶ UO 2 (C 2 O 4 )·3H 2 O + 2NaNO 3
Determine the limiting reactant and the percent yield of this reaction.
Na 2 C 2 O 4 is the limiting reactant. percent yield = 86.6%
How many molecules of C 2 H 4 Cl 2 can be prepared from 15 C 2 H 4 molecules and 8 Cl 2 molecules?
How many molecules of the sweetener saccharin can be prepared from 30 C atoms, 25 H atoms, 12 O atoms, 8 S atoms, and 14 N atoms?
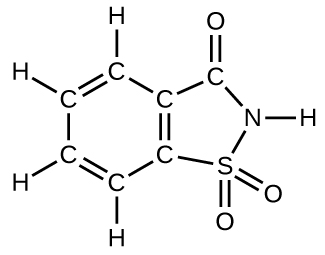
Only four molecules can be made.
The phosphorus pentoxide used to produce phosphoric acid for cola soft drinks is prepared by burning phosphorus in oxygen.
(a) What is the limiting reactant when 0.200 mol of P 4 and 0.200 mol of O 2 react according to
(b) Calculate the percent yield if 10.0 g of P 4 O 10 is isolated from the reaction.
Would you agree to buy 1 trillion (1,000,000,000,000) gold atoms for $5? Explain why or why not. Find the current price of gold at http://money.cnn.com/data/commodities/
This amount cannot be weighted by ordinary balances and is worthless.

Notification Switch
Would you like to follow the 'Chemistry' conversation and receive update notifications?