| << Chapter < Page | Chapter >> Page > |
Many medical tests require drawing a small amount of blood, for example to determine the amount of glucose in someone with diabetes or the hematocrit level in an athlete. This procedure can be easily done because of capillary action, the ability of a liquid to flow up a small tube against gravity, as shown in [link] . When your finger is pricked, a drop of blood forms and holds together due to surface tension—the unbalanced intermolecular attractions at the surface of the drop. Then, when the open end of a narrow-diameter glass tube touches the drop of blood, the adhesive forces between the molecules in the blood and those at the glass surface draw the blood up the tube. How far the blood goes up the tube depends on the diameter of the tube (and the type of fluid). A small tube has a relatively large surface area for a given volume of blood, which results in larger (relative) attractive forces, allowing the blood to be drawn farther up the tube. The liquid itself is held together by its own cohesive forces. When the weight of the liquid in the tube generates a downward force equal to the upward force associated with capillary action, the liquid stops rising.
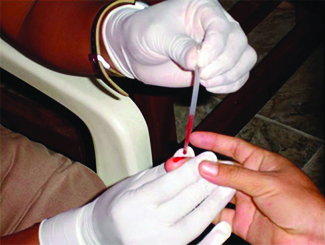
The intermolecular forces between molecules in the liquid state vary depending upon their chemical identities and result in corresponding variations in various physical properties. Cohesive forces between like molecules are responsible for a liquid’s viscosity (resistance to flow) and surface tension (elasticity of a liquid surface). Adhesive forces between the molecules of a liquid and different molecules composing a surface in contact with the liquid are responsible for phenomena such as surface wetting and capillary rise.
The test tubes shown here contain equal amounts of the specified motor oils. Identical metal spheres were dropped at the same time into each of the tubes, and a brief moment later, the spheres had fallen to the heights indicated in the illustration.
Rank the motor oils in order of increasing viscosity, and explain your reasoning:

Although steel is denser than water, a steel needle or paper clip placed carefully lengthwise on the surface of still water can be made to float. Explain at a molecular level how this is possible:

The water molecules have strong intermolecular forces of hydrogen bonding. The water molecules are thus attracted strongly to one another and exhibit a relatively large surface tension, forming a type of “skin” at its surface. This skin can support a bug or paper clip if gently placed on the water.
The surface tension and viscosity values for diethyl ether, acetone, ethanol, and ethylene glycol are shown here.
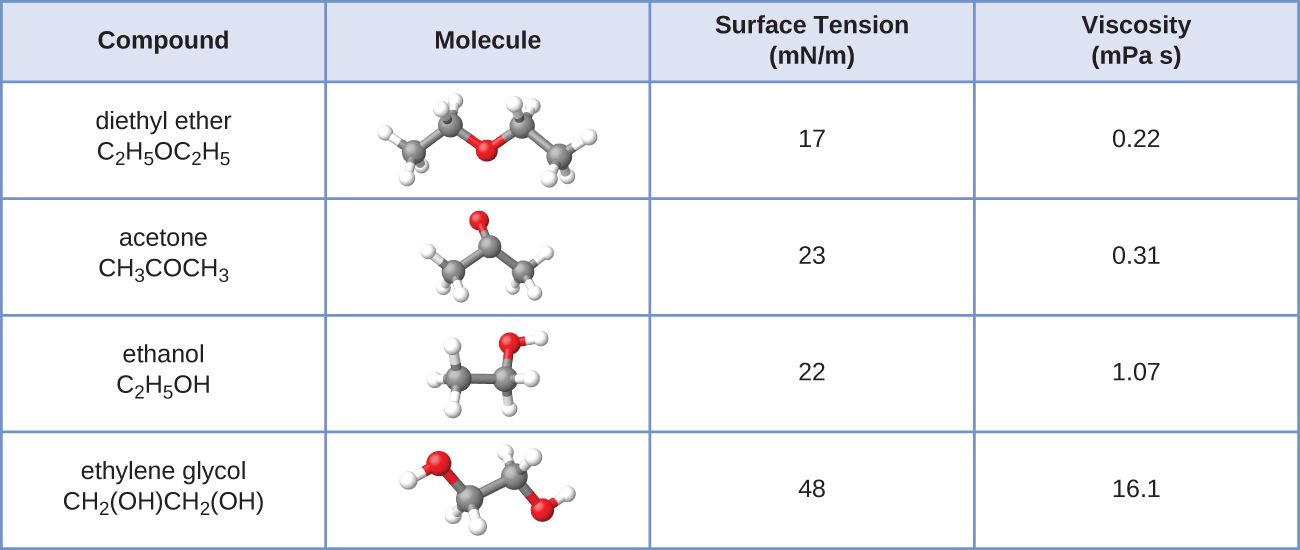
(a) Explain their differences in viscosity in terms of the size and shape of their molecules and their IMFs.
(b) Explain their differences in surface tension in terms of the size and shape of their molecules and their IMFs:
You may have heard someone use the figure of speech “slower than molasses in winter” to describe a process that occurs slowly. Explain why this is an apt idiom, using concepts of molecular size and shape, molecular interactions, and the effect of changing temperature.
Temperature has an effect on intermolecular forces: the higher the temperature, the greater the kinetic energies of the molecules and the greater the extent to which their intermolecular forces are overcome, and so the more fluid (less viscous) the liquid; the lower the temperature, the lesser the intermolecular forces are overcome, and so the less viscous the liquid.
It is often recommended that you let your car engine run idle to warm up before driving, especially on cold winter days. While the benefit of prolonged idling is dubious, it is certainly true that a warm engine is more fuel efficient than a cold one. Explain the reason for this.
The surface tension and viscosity of water at several different temperatures are given in this table.
| Water | Surface Tension (mN/m) | Viscosity (mPa s) |
|---|---|---|
| 0 °C | 75.6 | 1.79 |
| 20 °C | 72.8 | 1.00 |
| 60 °C | 66.2 | 0.47 |
| 100 °C | 58.9 | 0.28 |
(a) As temperature increases, what happens to the surface tension of water? Explain why this occurs, in terms of molecular interactions and the effect of changing temperature.
(b) As temperature increases, what happens to the viscosity of water? Explain why this occurs, in terms of molecular interactions and the effect of changing temperature.
(a) As the water reaches higher temperatures, the increased kinetic energies of its molecules are more effective in overcoming hydrogen bonding, and so its surface tension decreases. Surface tension and intermolecular forces are directly related. (b) The same trend in viscosity is seen as in surface tension, and for the same reason.
At 25 °C, how high will water rise in a glass capillary tube with an inner diameter of 0.63 mm? Refer to [link] for the required information.
Water rises in a glass capillary tube to a height of 17 cm. What is the diameter of the capillary tube?
9.5 10 −5 m

Notification Switch
Would you like to follow the 'Chemistry' conversation and receive update notifications?