| << Chapter < Page | Chapter >> Page > |
Finally, here is one more example showing how to calculate molar mass from effusion rate data.
Plug in known data:
Solve:
The gas could well be CH 4 , the only gas with this molar mass.
163 g/mol
Gaseous diffusion has been used to produce enriched uranium for use in nuclear power plants and weapons. Naturally occurring uranium contains only 0.72% of 235 U, the kind of uranium that is “fissile,” that is, capable of sustaining a nuclear fission chain reaction. Nuclear reactors require fuel that is 2–5% 235 U, and nuclear bombs need even higher concentrations. One way to enrich uranium to the desired levels is to take advantage of Graham’s law. In a gaseous diffusion enrichment plant, uranium hexafluoride (UF 6 , the only uranium compound that is volatile enough to work) is slowly pumped through large cylindrical vessels called diffusers, which contain porous barriers with microscopic openings. The process is one of diffusion because the other side of the barrier is not evacuated. The 235 UF 6 molecules have a higher average speed and diffuse through the barrier a little faster than the heavier 238 UF 6 molecules. The gas that has passed through the barrier is slightly enriched in 235 UF 6 and the residual gas is slightly depleted. The small difference in molecular weights between 235 UF 6 and 238 UF 6 only about 0.4% enrichment, is achieved in one diffuser ( [link] ). But by connecting many diffusers in a sequence of stages (called a cascade), the desired level of enrichment can be attained.
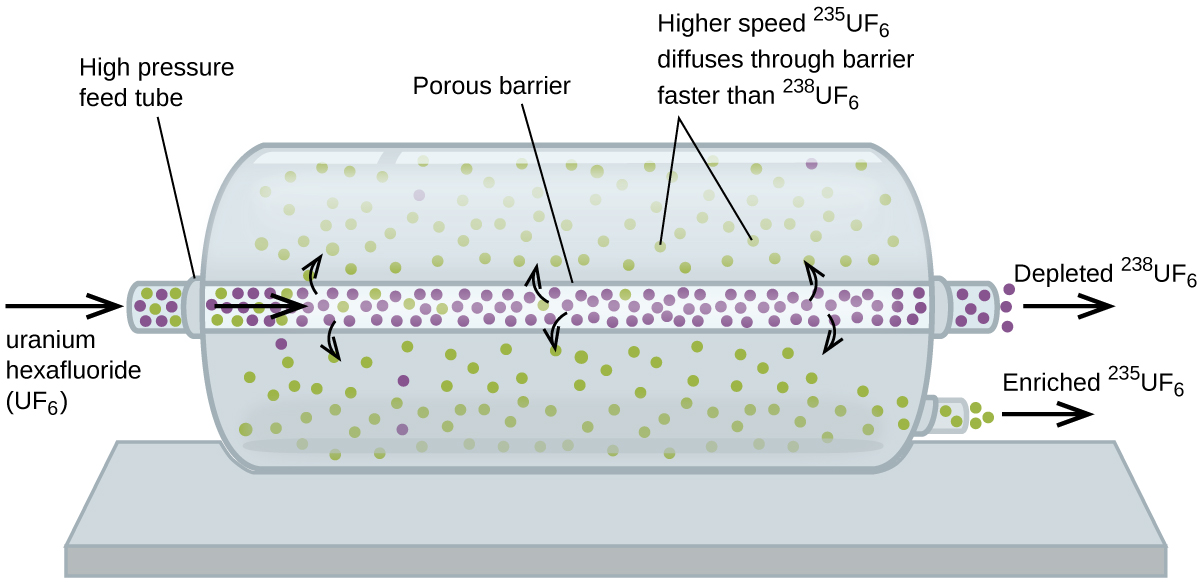
The large scale separation of gaseous 235 UF 6 from 238 UF 6 was first done during the World War II, at the atomic energy installation in Oak Ridge, Tennessee, as part of the Manhattan Project (the development of the first atomic bomb). Although the theory is simple, this required surmounting many daunting technical challenges to make it work in practice. The barrier must have tiny, uniform holes (about 10 –6 cm in diameter) and be porous enough to produce high flow rates. All materials (the barrier, tubing, surface coatings, lubricants, and gaskets) need to be able to contain, but not react with, the highly reactive and corrosive UF 6 .
Because gaseous diffusion plants require very large amounts of energy (to compress the gas to the high pressures required and drive it through the diffuser cascade, to remove the heat produced during compression, and so on), it is now being replaced by gas centrifuge technology, which requires far less energy. A current hot political issue is how to deny this technology to Iran, to prevent it from producing enough enriched uranium for them to use to make nuclear weapons.
Gaseous atoms and molecules move freely and randomly through space. Diffusion is the process whereby gaseous atoms and molecules are transferred from regions of relatively high concentration to regions of relatively low concentration. Effusion is a similar process in which gaseous species pass from a container to a vacuum through very small orifices. The rates of effusion of gases are inversely proportional to the square roots of their densities or to the square roots of their atoms/molecules’ masses (Graham’s law).
A balloon filled with helium gas is found to take 6 hours to deflate to 50% of its original volume. How long will it take for an identical balloon filled with the same volume of hydrogen gas (instead of helium) to decrease its volume by 50%?
4.2 hours
Explain why the numbers of molecules are not identical in the left- and right-hand bulbs shown in the center illustration of [link] .
Starting with the definition of rate of effusion and Graham’s finding relating rate and molar mass, show how to derive the Graham’s law equation, relating the relative rates of effusion for two gases to their molecular masses.
Effusion can be defined as the process by which a gas escapes through a pinhole into a vacuum. Graham’s law states that with a mixture of two gases A and B:
Both A and B are in the same container at the same temperature, and therefore will have the same kinetic energy:
Therefore,
Heavy water, D 2 O (molar mass = 20.03 g mol –1 ), can be separated from ordinary water, H 2 O (molar mass = 18.01), as a result of the difference in the relative rates of diffusion of the molecules in the gas phase. Calculate the relative rates of diffusion of H 2 O and D 2 O.
Which of the following gases diffuse more slowly than oxygen? F 2 , Ne, N 2 O, C 2 H 2 , NO, Cl 2 , H 2 S
F 2 , N 2 O, Cl 2 , H 2 S
During the discussion of gaseous diffusion for enriching uranium, it was claimed that 235 UF 6 diffuses 0.4% faster than 238 UF 6 . Show the calculation that supports this value. The molar mass of 235 UF 6 = 235.043930 + 6 18.998403 = 349.034348 g/mol, and the molar mass of 238 UF 6 = 238.050788 + 6 18.998403 = 352.041206 g/mol.
Calculate the relative rate of diffusion of 1 H 2 (molar mass 2.0 g/mol) compared to that of 2 H 2 (molar mass 4.0 g/mol) and the relative rate of diffusion of O 2 (molar mass 32 g/mol) compared to that of O 3 (molar mass 48 g/mol).
1.4; 1.2
A gas of unknown identity diffuses at a rate of 83.3 mL/s in a diffusion apparatus in which carbon dioxide diffuses at the rate of 102 mL/s. Calculate the molecular mass of the unknown gas.
When two cotton plugs, one moistened with ammonia and the other with hydrochloric acid, are simultaneously inserted into opposite ends of a glass tube that is 87.0 cm long, a white ring of NH
4 Cl forms where gaseous NH
3 and gaseous HCl first come into contact. (Hint: Calculate the rates of diffusion for both NH
3 and HCl, and find out how much faster NH
3 diffuses than HCl.)
At approximately what distance from the ammonia moistened plug does this occur?
51.7 cm

Notification Switch
Would you like to follow the 'Chemistry' conversation and receive update notifications?