| << Chapter < Page | Chapter >> Page > |
Aluminum is a very good reducing agent and may replace other reducing agents in the isolation of certain metals from their oxides. Although more expensive than reduction by carbon, aluminum is important in the isolation of Mo, W, and Cr from their oxides.
The metallic members of group 14 are tin, lead, and flerovium. Carbon is a typical nonmetal. The remaining elements of the group, silicon and germanium, are examples of semimetals or metalloids. Tin and lead form the stable divalent cations, Sn 2+ and Pb 2+ , with oxidation states two below the group oxidation state of 4+. The stability of this oxidation state is a consequence of the inert pair effect. Tin and lead also form covalent compounds with a formal 4+-oxidation state. For example, SnCl 4 and PbCl 4 are low-boiling covalent liquids.
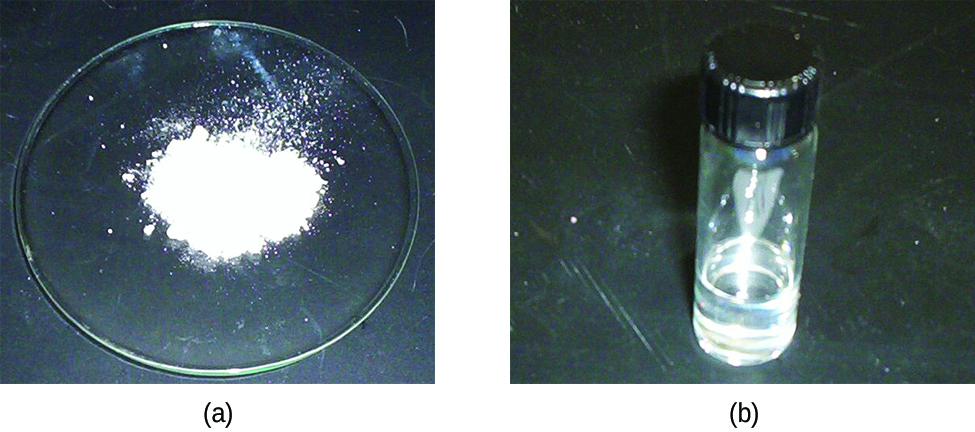
Tin reacts readily with nonmetals and acids to form tin(II) compounds (indicating that it is more easily oxidized than hydrogen) and with nonmetals to form either tin(II) or tin(IV) compounds (shown in [link] ), depending on the stoichiometry and reaction conditions. Lead is less reactive. It is only slightly easier to oxidize than hydrogen, and oxidation normally requires a hot concentrated acid.
Many of these elements exist as allotropes. Allotropes are two or more forms of the same element in the same physical state with different chemical and physical properties. There are two common allotropes of tin. These allotropes are grey (brittle) tin and white tin. As with other allotropes, the difference between these forms of tin is in the arrangement of the atoms. White tin is stable above 13.2 °C and is malleable like other metals. At low temperatures, gray tin is the more stable form. Gray tin is brittle and tends to break down to a powder. Consequently, articles made of tin will disintegrate in cold weather, particularly if the cold spell is lengthy. The change progresses slowly from the spot of origin, and the gray tin that is first formed catalyzes further change. In a way, this effect is similar to the spread of an infection in a plant or animal body, leading people to call this process tin disease or tin pest.
The principal use of tin is in the coating of steel to form tin plate-sheet iron, which constitutes the tin in tin cans. Important tin alloys are bronze (Cu and Sn) and solder (Sn and Pb). Lead is important in the lead storage batteries in automobiles.
Bismuth , the heaviest member of group 15, is a less reactive metal than the other representative metals. It readily gives up three of its five valence electrons to active nonmetals to form the tri-positive ion, Bi 3+ . It forms compounds with the group oxidation state of 5+ only when treated with strong oxidizing agents. The stability of the 3+-oxidation state is another example of the inert pair effect.
This section focuses on the periodicity of the representative elements. These are the elements where the electrons are entering the s and p orbitals. The representative elements occur in groups 1, 2, and 12–18. These elements are representative metals, metalloids, and nonmetals. The alkali metals (group 1) are very reactive, readily form ions with a charge of 1+ to form ionic compounds that are usually soluble in water, and react vigorously with water to form hydrogen gas and a basic solution of the metal hydroxide. The outermost electrons of the alkaline earth metals (group 2) are more difficult to remove than the outer electron of the alkali metals, leading to the group 2 metals being less reactive than those in group 1. These elements easily form compounds in which the metals exhibit an oxidation state of 2+. Zinc, cadmium, and mercury (group 12) commonly exhibit the group oxidation state of 2+ (although mercury also exhibits an oxidation state of 1+ in compounds that contain Aluminum, gallium, indium, and thallium (group 13) are easier to oxidize than is hydrogen. Aluminum, gallium, and indium occur with an oxidation state 3+ (however, thallium also commonly occurs as the Tl + ion). Tin and lead form stable divalent cations and covalent compounds in which the metals exhibit the 4+-oxidation state.

Notification Switch
Would you like to follow the 'Chemistry' conversation and receive update notifications?