| << Chapter < Page | Chapter >> Page > |
Formaldehyde, an aldehyde with the formula HCHO, is a colorless gas with a pungent and irritating odor. It is sold in an aqueous solution called formalin, which contains about 37% formaldehyde by weight. Formaldehyde causes coagulation of proteins, so it kills bacteria (and any other living organism) and stops many of the biological processes that cause tissue to decay. Thus, formaldehyde is used for preserving tissue specimens and embalming bodies. It is also used to sterilize soil or other materials. Formaldehyde is used in the manufacture of Bakelite, a hard plastic having high chemical and electrical resistance.
Dimethyl ketone, CH 3 COCH 3 , commonly called acetone, is the simplest ketone. It is made commercially by fermenting corn or molasses, or by oxidation of 2-propanol. Acetone is a colorless liquid. Among its many uses are as a solvent for lacquer (including fingernail polish), cellulose acetate, cellulose nitrate, acetylene, plastics, and varnishes; as a paint and varnish remover; and as a solvent in the manufacture of pharmaceuticals and chemicals.
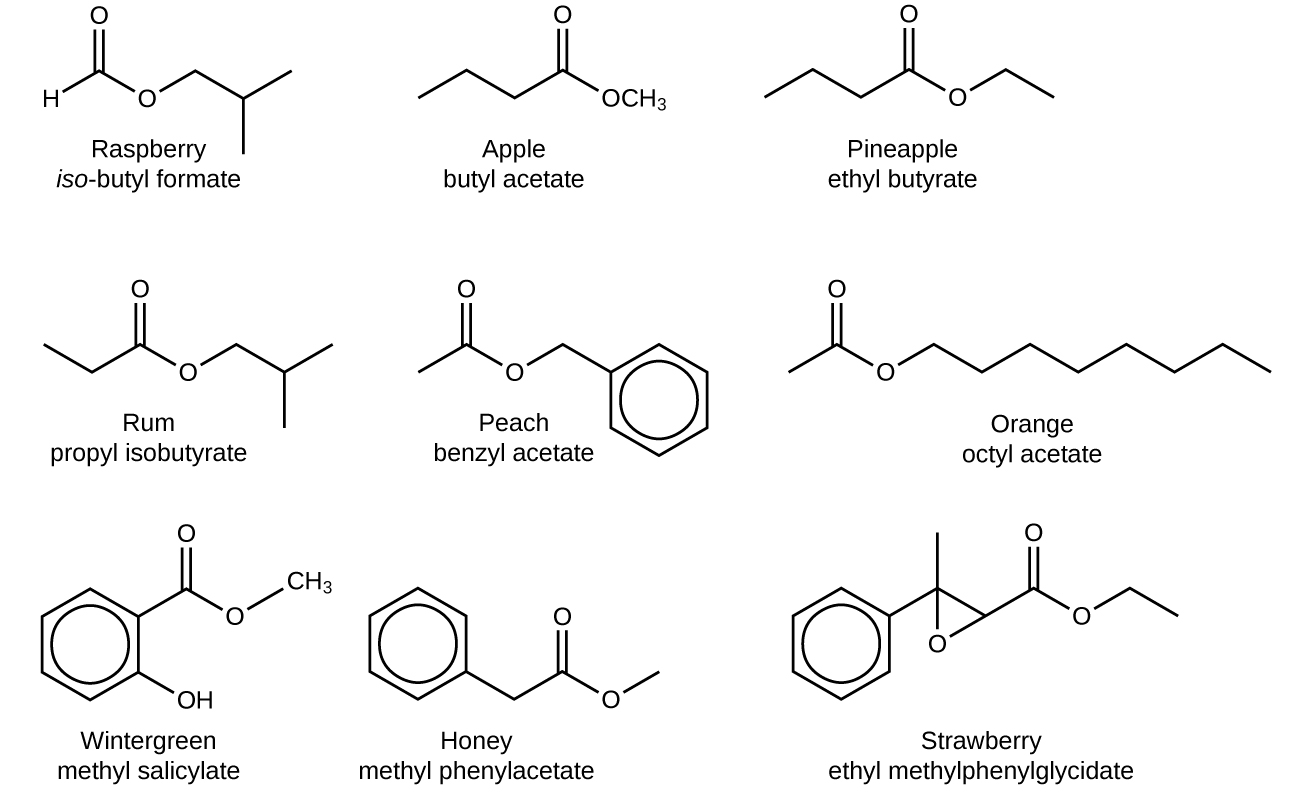
The odor of vinegar is caused by the presence of acetic acid, a carboxylic acid, in the vinegar. The odor of ripe bananas and many other fruits is due to the presence of esters, compounds that can be prepared by the reaction of a carboxylic acid with an alcohol. Because esters do not have hydrogen bonds between molecules, they have lower vapor pressures than the alcohols and carboxylic acids from which they are derived (see [link] ).
Both carboxylic acids and esters contain a carbonyl group with a second oxygen atom bonded to the carbon atom in the carbonyl group by a single bond. In a carboxylic acid, the second oxygen atom also bonds to a hydrogen atom. In an ester, the second oxygen atom bonds to another carbon atom. The names for carboxylic acids and esters include prefixes that denote the lengths of the carbon chains in the molecules and are derived following nomenclature rules similar to those for inorganic acids and salts (see these examples):

The functional groups for an acid and for an ester are shown in red in these formulas.
The hydrogen atom in the functional group of a carboxylic acid will react with a base to form an ionic salt:
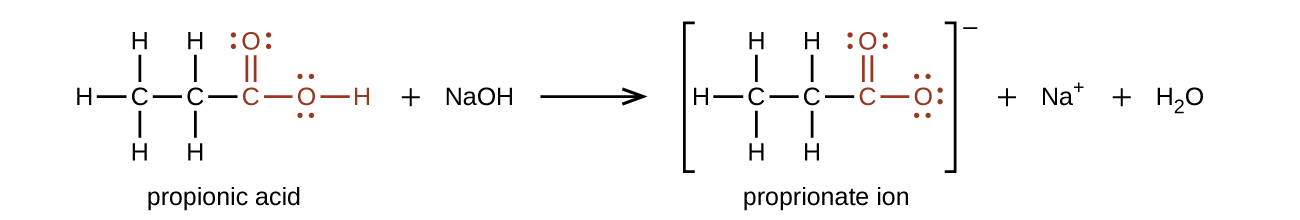
Carboxylic acids are weak acids (see the chapter on acids and bases), meaning they are not 100% ionized in water. Generally only about 1% of the molecules of a carboxylic acid dissolved in water are ionized at any given time. The remaining molecules are undissociated in solution.
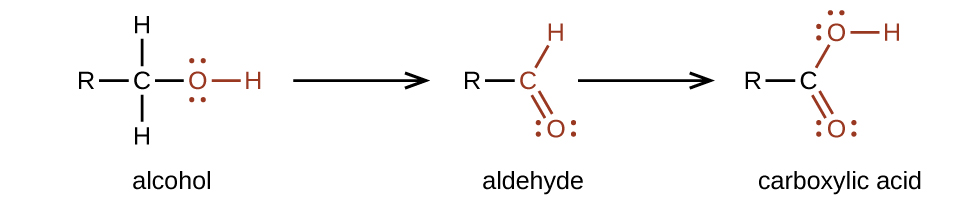
We prepare carboxylic acids by the oxidation of aldehydes or alcohols whose –OH functional group is located on the carbon atom at the end of the chain of carbon atoms in the alcohol:
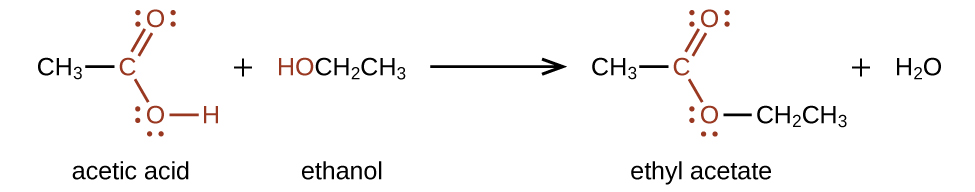
Esters are produced by the reaction of acids with alcohols. For example, the ester ethyl acetate, CH 3 CO 2 CH 2 CH 3 , is formed when acetic acid reacts with ethanol:
The simplest carboxylic acid is formic acid, HCO 2 H, known since 1670. Its name comes from the Latin word formicus , which means “ant”; it was first isolated by the distillation of red ants. It is partially responsible for the pain and irritation of ant and wasp stings, and is responsible for a characteristic odor of ants that can be sometimes detected in their nests.

Notification Switch
Would you like to follow the 'Chemistry' conversation and receive update notifications?