| << Chapter < Page | Chapter >> Page > |
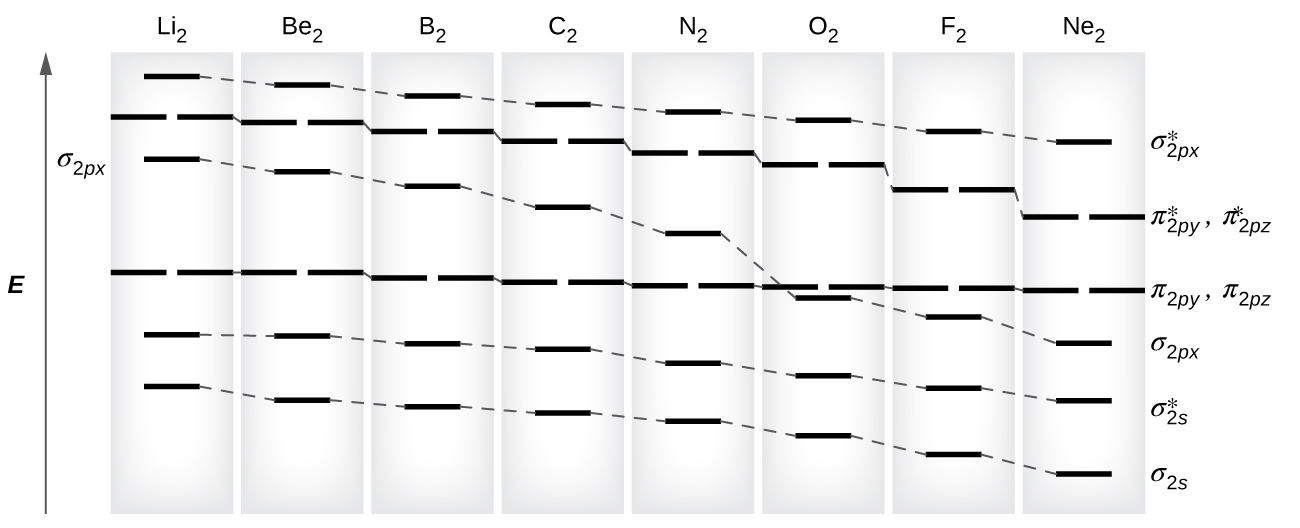
As we saw in valence bond theory, σ bonds are generally more stable than π bonds formed from degenerate atomic orbitals. Similarly, in molecular orbital theory, σ orbitals are usually more stable than π orbitals. However, this is not always the case. The MOs for the valence orbitals of the second period are shown in [link] . Looking at Ne 2 molecular orbitals, we see that the order is consistent with the generic diagram shown in the previous section. However, for atoms with three or fewer electrons in the p orbitals (Li through N) we observe a different pattern, in which the σ p orbital is higher in energy than the π p set. Obtain the molecular orbital diagram for a homonuclear diatomic ion by adding or subtracting electrons from the diagram for the neutral molecule.
You can practice labeling and filling molecular orbitals with this interactive tutorial from the University of Sydney.
This switch in orbital ordering occurs because of a phenomenon called s-p mixing . s-p mixing does not create new orbitals; it merely influences the energies of the existing molecular orbitals. The σ s wavefunction mathematically combines with the σ p wavefunction, with the result that the σ s orbital becomes more stable, and the σ p orbital becomes less stable ( [link] ). Similarly, the antibonding orbitals also undergo s-p mixing, with the σ s* becoming more stable and the σ p* becoming less stable.
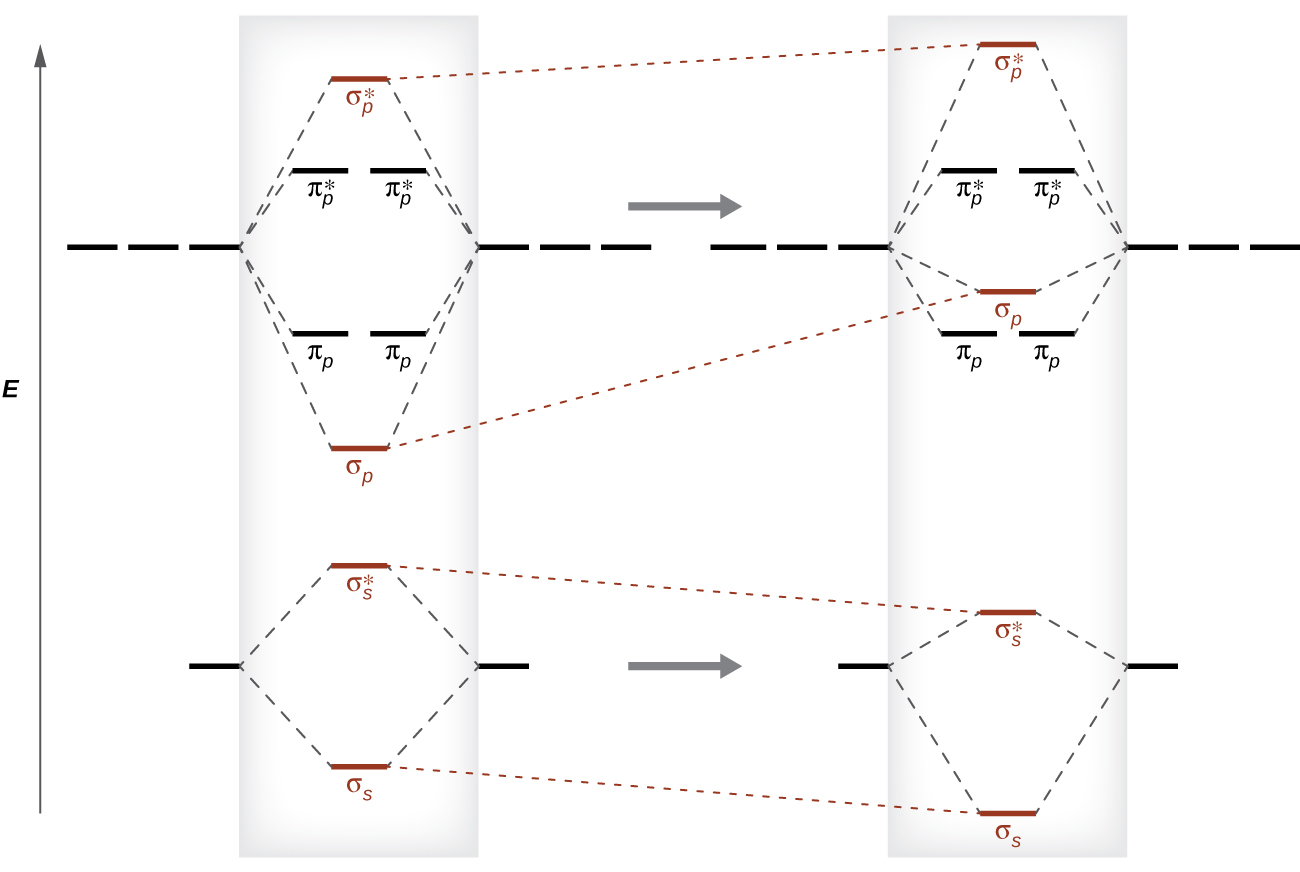
s-p mixing occurs when the s and p orbitals have similar energies. When a single p orbital contains a pair of electrons, the act of pairing the electrons raises the energy of the orbital. Thus the 2 p orbitals for O, F, and Ne are higher in energy than the 2 p orbitals for Li, Be, B, C, and N. Because of this, O 2 , F 2 , and N 2 only have negligible s-p mixing (not sufficient to change the energy ordering), and their MO diagrams follow the normal pattern, as shown in [link] . All of the other period 2 diatomic molecules do have s-p mixing, which leads to the pattern where the σ p orbital is raised above the π p set.
Using the MO diagrams shown in [link] , we can add in the electrons and determine the molecular electron configuration and bond order for each of the diatomic molecules. As shown in [link] , Be 2 and Ne 2 molecules would have a bond order of 0, and these molecules do not exist.
| Electron Configuration and Bond Order for Molecular Orbitals in Homonuclear Diatomic Molecules of Period Two Elements | ||
|---|---|---|
| Molecule | Electron Configuration | Bond Order |
| Li 2 | 1 | |
| Be 2 (unstable) | 0 | |
| B 2 | 1 | |
| C 2 | 2 | |
| N 2 | 3 | |
| O 2 | 2 | |
| F 2 | 1 | |
| Ne 2 (unstable) | 0 |

Notification Switch
Would you like to follow the 'Chemistry' conversation and receive update notifications?