| << Chapter < Page | Chapter >> Page > |
In 1965, scientists at Michigan State University discovered that there was a platinum complex that inhibited cell division in certain microorganisms. Later work showed that the complex was cis -diaminedichloroplatinum(II), [Pt(NH 3 ) 2 (Cl) 2 ], and that the trans isomer was not effective. The inhibition of cell division indicated that this square planar compound could be an anticancer agent. In 1978, the US Food and Drug Administration approved this compound, known as cisplatin, for use in the treatment of certain forms of cancer. Since that time, many similar platinum compounds have been developed for the treatment of cancer. In all cases, these are the cis isomers and never the trans isomers. The diamine (NH 3 ) 2 portion is retained with other groups, replacing the dichloro [(Cl) 2 ] portion. The newer drugs include carboplatin, oxaliplatin, and satraplatin.
The transition elements and main group elements can form coordination compounds, or complexes, in which a central metal atom or ion is bonded to one or more ligands by coordinate covalent bonds. Ligands with more than one donor atom are called polydentate ligands and form chelates. The common geometries found in complexes are tetrahedral and square planar (both with a coordination number of four) and octahedral (with a coordination number of six). Cis and trans configurations are possible in some octahedral and square planar complexes. In addition to these geometrical isomers, optical isomers (molecules or ions that are mirror images but not superimposable) are possible in certain octahedral complexes. Coordination complexes have a wide variety of uses including oxygen transport in blood, water purification, and pharmaceutical use.
Indicate the coordination number for the central metal atom in each of the following coordination compounds:
(a) [Pt(H 2 O) 2 Br 2 ]
(b) [Pt(NH 3 )(py)(Cl)(Br)] (py = pyridine, C 5 H 5 N)
(c) [Zn(NH 3 ) 2 Cl 2 ]
(d) [Zn(NH 3 )(py)(Cl)(Br)]
(e) [Ni(H 2 O) 4 Cl 2 ]
(f) [Fe(en) 2 (CN) 2 ] + (en = ethylenediamine, C 2 H 8 N 2 )
Give the coordination numbers and write the formulas for each of the following, including all isomers where appropriate:
(a) tetrahydroxozincate(II) ion (tetrahedral)
(b) hexacyanopalladate(IV) ion
(c) dichloroaurate(I) ion (note that aurum is Latin for "gold")
(d) diaminedichloroplatinum(II)
(e) potassium diaminetetrachlorochromate(III)
(f) hexaaminecobalt(III) hexacyanochromate(III)
(g) dibromobis(ethylenediamine) cobalt(III) nitrate
(a) 4, [Zn(OH) 4 ] 2− ; (b) 6, [Pd(CN) 6 ] 2− ; (c) 2, [AuCl 2 ] − ; (d) 4, [Pt(NH 3 ) 2 Cl 2 ]; (e) 6, K[Cr(NH 3 ) 2 Cl 4 ]; (f) 6, [Co(NH 3 ) 6 ][Cr(CN) 6 ]; (g) 6, [Co(en) 2 Br 2 ]NO 3
Give the coordination number for each metal ion in the following compounds:
(a) [Co(CO 3 ) 3 ] 3− (note that CO 3 2− is bidentate in this complex)
(b) [Cu(NH 3 ) 4 ] 2+
(c) [Co(NH 3 ) 4 Br 2 ] 2 (SO 4 ) 3
(d) [Pt(NH 3 ) 4 ][PtCl 4 ]
(e) [Cr(en) 3 ](NO 3 ) 3
(f) [Pd(NH 3 ) 2 Br 2 ] (square planar)
(g) K 3 [Cu(Cl) 5 ]
(h) [Zn(NH 3 ) 2 Cl 2 ]
Sketch the structures of the following complexes. Indicate any cis , trans , and optical isomers.
(a) [Pt(H 2 O) 2 Br 2 ] (square planar)
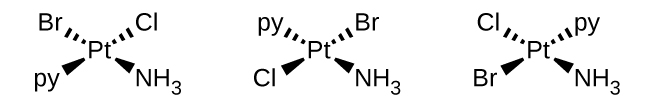
(b) [Pt(NH 3 )(py)(Cl)(Br)] (square planar, py = pyridine, C 5 H 5 N)
(c) [Zn(NH 3 ) 3 Cl] + (tetrahedral)
(d) [Pt(NH 3 ) 3 Cl] + (square planar)
(e) [Ni(H 2 O) 4 Cl 2 ]
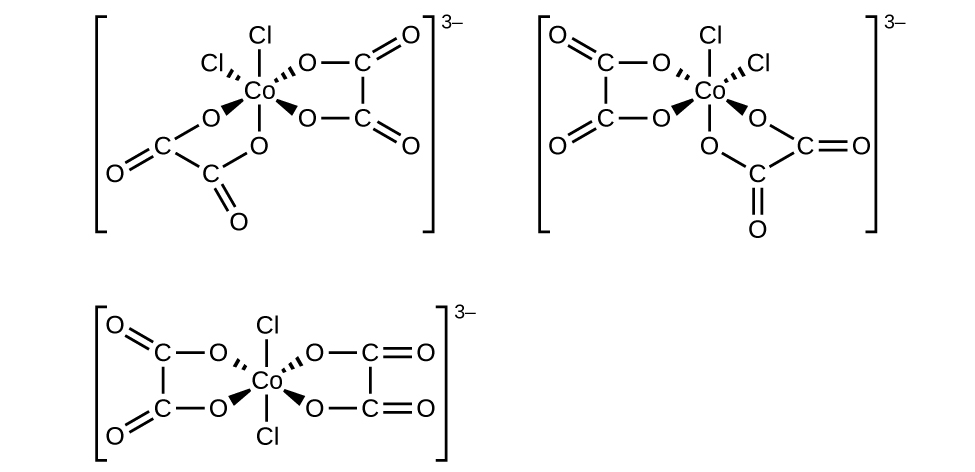
(f) [Co(C 2 O 4 ) 2 Cl 2 ] 3− (note that is the bidentate oxalate ion,
(a) [Pt(H
2 O)
2 Br
2 ]:
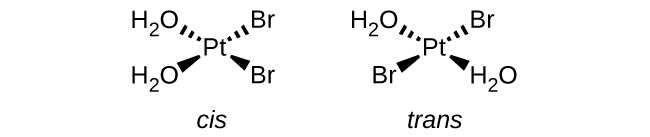
(b) [Pt(NH
3 )(py)(Cl)(Br)]:
(c) [Zn(NH
3 )
3 Cl]
+ :
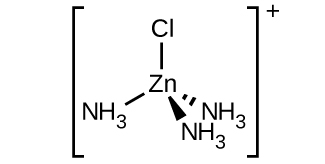
(d) [Pt(NH
3 )
3 Cl]
+ :
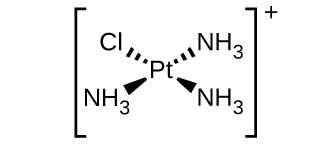
(e) [Ni(H
2 O)
4 Cl
2 ]:
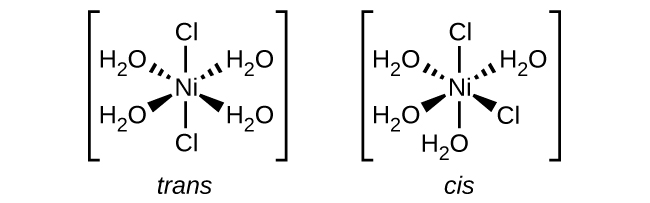
(f) [Co(C
2 O
4 )
2 Cl
2 ]
3− :
Draw diagrams for any cis , trans , and optical isomers that could exist for the following (en is ethylenediamine):
(a) [Co(en) 2 (NO 2 )Cl] +
(b) [Co(en) 2 Cl 2 ] +
(c) [Pt(NH 3 ) 2 Cl 4 ]
(d) [Cr(en) 3 ] 3+
(e) [Pt(NH 3 ) 2 Cl 2 ]
Name each of the compounds or ions given in [link] , including the oxidation state of the metal.
(a) tricarbonatocobaltate(III) ion; (b) tetraaminecopper(II) ion; (c) tetraaminedibromocobalt(III) sulfate; (d) tetraamineplatinum(II) tetrachloroplatinate(II); (e) tris- (ethylenediamine)chromium(III) nitrate; (f) diaminedibromopalladium(II); (g) potassium pentachlorocuprate(II); (h) diaminedichlorozinc(II)
Name each of the compounds or ions given in [link] .
Specify whether the following complexes have isomers.
(a) tetrahedral [Ni(CO) 2 (Cl) 2 ]
(b) trigonal bipyramidal [Mn(CO) 4 NO]
(c) [Pt(en) 2 Cl 2 ]Cl 2
(a) none; (b) none; (c) The two Cl ligands can be cis or trans . When they are cis , there will also be an optical isomer.
Predict whether the carbonate ligand will coordinate to a metal center as a monodentate, bidentate, or tridentate ligand.
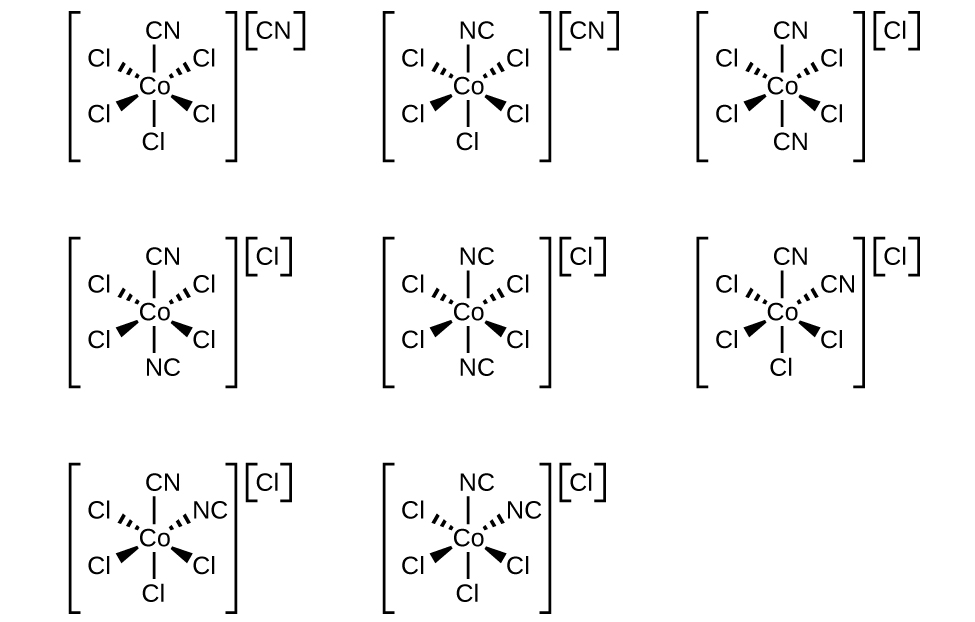
Draw the geometric, linkage, and ionization isomers for [CoCl 5 CN][CN].

Notification Switch
Would you like to follow the 'Chemistry' conversation and receive update notifications?