| << Chapter < Page | Chapter >> Page > |
Calcium hydrogen carbonate, Ca(HCO 3 ) 2 , is soluble, so limestone and marble objects slowly dissolve in acid rain.
If we add calcium carbonate to a concentrated acid, hydronium ion reacts with the carbonate ion according to the equation:
(Acid rain is usually not sufficiently acidic to cause this reaction; however, laboratory acids are.) The solution may become saturated with the weak electrolyte carbonic acid, which is unstable, and carbon dioxide gas can be evolved:
These reactions decrease the carbonate ion concentration, and additional calcium carbonate dissolves. If enough acid is present, the concentration of carbonate ion is reduced to such a low level that the reaction quotient for the dissolution of calcium carbonate remains less than the solubility product of calcium carbonate, even after all of the calcium carbonate has dissolved.
Reaction (1):
Reaction (2):
To prevent the formation of solid Mg(OH) 2 , we must adjust the concentration of OH – so that the reaction quotient for Equation (1), Q = [Mg 2+ ][OH – ] 2 , is less than K sp for Mg(OH) 2 . (To simplify the calculation, we determine the concentration of OH – when Q = K sp .) [OH – ] can be reduced by the addition of which shifts Reaction (2) to the left and reduces [OH – ].
We determine the [OH – ] at which Q = K sp when [Mg 2+ ] = 0.10 M:
Solid Mg(OH) 2 will not form in this solution when [OH – ] is less than 1.2 10 –5 M .
We calculate the needed to decrease [OH – ] to 1.2 10 –5 M when [NH 3 ] = 0.10.
When equals 0.19 M , [OH – ] will be 9.4 10 –6 M . Any greater than 0.19 M will reduce [OH – ] below 9.4 10 –6 M and prevent the formation of Mg(OH) 2 .
and calculate the concentration of hydronium ion required to prevent the precipitation of ZnS in a solution that is 0.050 M in Zn 2+ and saturated with H 2 S (0.10 M H 2 S).
([S 2– ] is less than 2 10 –26 M and precipitation of ZnS does not occur.)
Therefore, precise calculations of the solubility of solids from the solubility product are limited to cases in which the only significant reaction occurring when the solid dissolves is the formation of its ions.
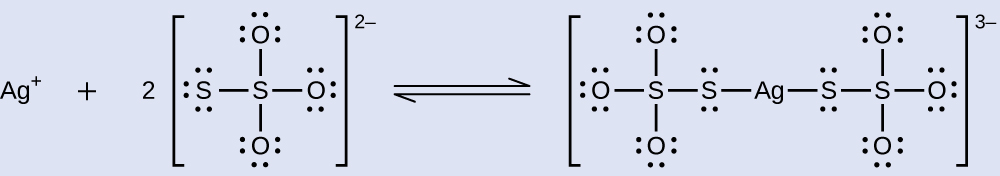
What mass of Na 2 S 2 O 3 is required to prepare 1.00 L of a solution that will dissolve 1.00 g of AgBr by the formation of

Notification Switch
Would you like to follow the 'Chemistry' conversation and receive update notifications?