| << Chapter < Page | Chapter >> Page > |
In summary, it takes six turns of the Calvin cycle to fix six carbon atoms from CO 2 . These six turns require energy input from 12 ATP molecules and 12 NADPH molecules in the reduction step and 6 ATP molecules in the regeneration step.
The following is a link to an animation of the Calvin cycle. Click Stage 1, Stage 2, and then Stage 3 to see G3P and ATP regenerate to form RuBP.
However, as with all biochemical pathways, a variety of conditions leads to varied adaptations that affect the basic pattern. Photosynthesis in dry-climate plants ( [link] ) has evolved with adaptations that conserve water. In the harsh dry heat, every drop of water and precious energy must be used to survive. Two adaptations have evolved in such plants. In one form, a more efficient use of CO 2 allows plants to photosynthesize even when CO 2 is in short supply, as when the stomata are closed on hot days. The other adaptation performs preliminary reactions of the Calvin cycle at night, because opening the stomata at this time conserves water due to cooler temperatures. In addition, this adaptation has allowed plants to carry out low levels of photosynthesis without opening stomata at all, an extreme mechanism to face extremely dry periods.

The two parts of photosynthesis—the light-dependent reactions and the Calvin cycle—have been described, as they take place in chloroplasts. However, prokaryotes, such as cyanobacteria, lack membrane-bound organelles. Prokaryotic photosynthetic autotrophic organisms have infoldings of the plasma membrane for chlorophyll attachment and photosynthesis ( [link] ). It is here that organisms like cyanobacteria can carry out photosynthesis.
Living things access energy by breaking down carbohydrate molecules. However, if plants make carbohydrate molecules, why would they need to break them down? Carbohydrates are storage molecules for energy in all living things. Although energy can be stored in molecules like ATP, carbohydrates are much more stable and efficient reservoirs for chemical energy. Photosynthetic organisms also carry out the reactions of respiration to harvest the energy that they have stored in carbohydrates, for example, plants have mitochondria in addition to chloroplasts.
You may have noticed that the overall reaction for photosynthesis:
is the reverse of the overall reaction for cellular respiration:
Photosynthesis produces oxygen as a byproduct, and respiration produces carbon dioxide as a byproduct.
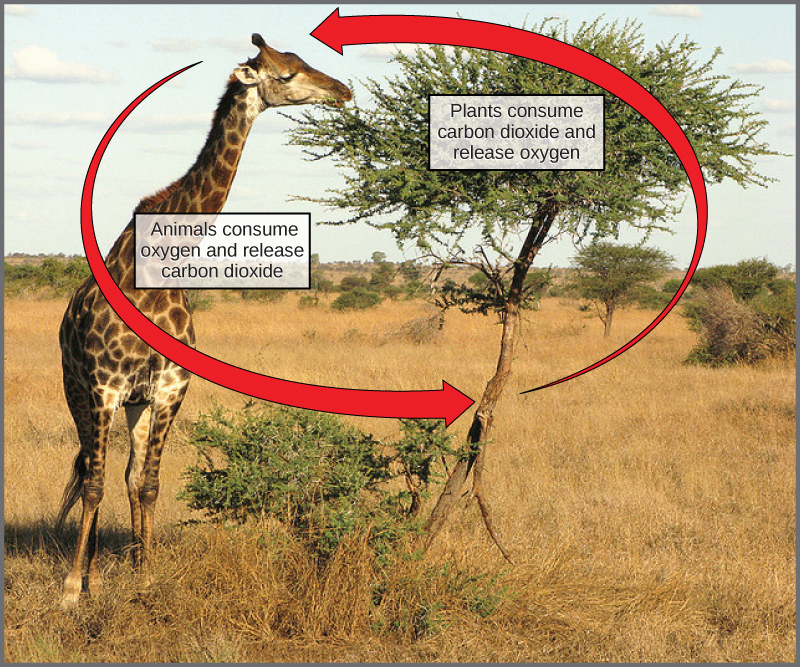
In nature, there is no such thing as waste. Every single atom of matter is conserved, recycling indefinitely. Substances change form or move from one type of molecule to another, but never disappear ( [link] ).
CO 2 is no more a form of waste produced by respiration than oxygen is a waste product of photosynthesis. Both are byproducts of reactions that move on to other reactions. Photosynthesis absorbs energy to build carbohydrates in chloroplasts, and aerobic cellular respiration releases energy by using oxygen to break down carbohydrates. Both organelles use electron transport chains to generate the energy necessary to drive other reactions. Photosynthesis and cellular respiration function in a biological cycle, allowing organisms to access life-sustaining energy that originates millions of miles away in a star.
Using the energy carriers formed in the first stage of photosynthesis, the Calvin cycle reactions fix CO 2 from the environment to build carbohydrate molecules. An enzyme, RuBisCO, catalyzes the fixation reaction, by combining CO 2 with RuBP. The resulting six-carbon compound is broken down into two three-carbon compounds, and the energy in ATP and NADPH is used to convert these molecules into G3P. One of the three-carbon molecules of G3P leaves the cycle to become a part of a carbohydrate molecule. The remaining G3P molecules stay in the cycle to be formed back into RuBP, which is ready to react with more CO 2 . Photosynthesis forms a balanced energy cycle with the process of cellular respiration. Plants are capable of both photosynthesis and cellular respiration, since they contain both chloroplasts and mitochondria.

Notification Switch
Would you like to follow the 'Concepts of biology' conversation and receive update notifications?