| << Chapter < Page | Chapter >> Page > |
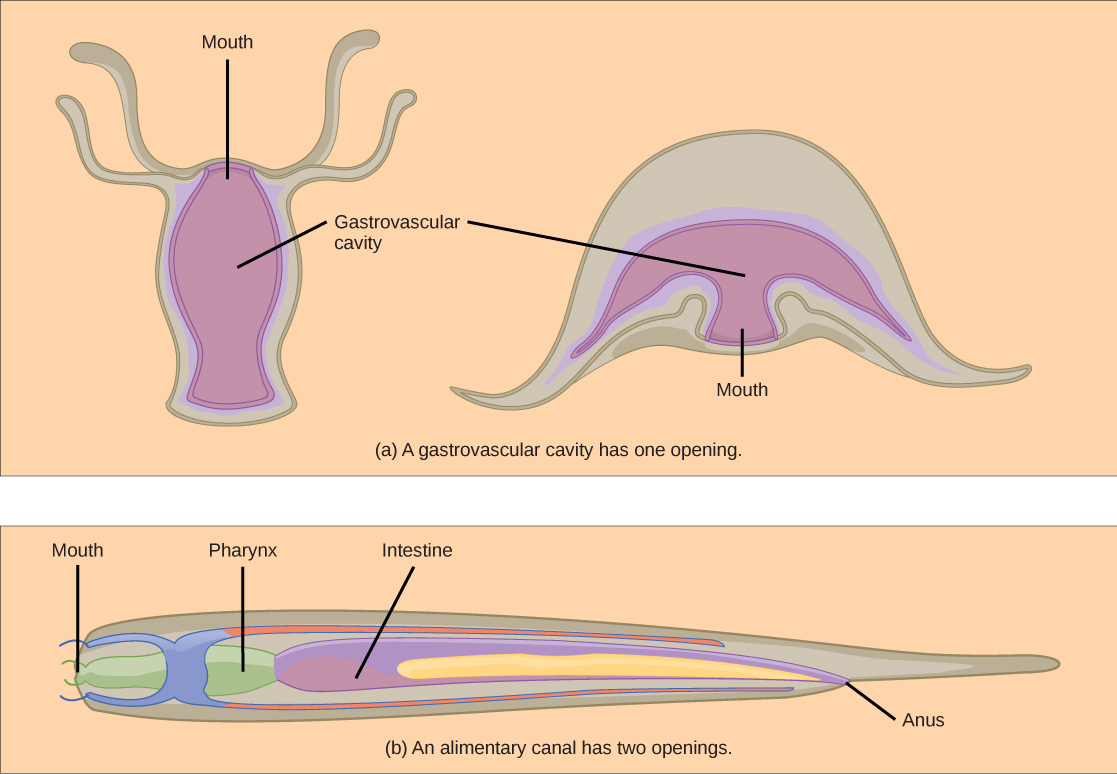
Animals have evolved different types of digestive systems to aid in the digestion of the different foods they consume. The simplest example is that of a gastrovascular cavity and is found in organisms with only one opening for digestion. Platyhelminthes (flatworms), Ctenophora (comb jellies), and Cnidaria (coral, jelly fish, and sea anemones) use this type of digestion. Gastrovascular cavities, as shown in [link] a , are typically a blind tube or cavity with only one opening, the “mouth”, which also serves as an “anus”. Ingested material enters the mouth and passes through a hollow, tubular cavity. Cells within the cavity secrete digestive enzymes that break down the food. The food particles are engulfed by the cells lining the gastrovascular cavity.
The alimentary canal , shown in [link] b , is a more advanced system: it consists of one tube with a mouth at one end and an anus at the other. Earthworms are an example of an animal with an alimentary canal. Once the food is ingested through the mouth, it passes through the esophagus and is stored in an organ called the crop; then it passes into the gizzard where it is churned and digested. From the gizzard, the food passes through the intestine, the nutrients are absorbed, and the waste is eliminated as feces, called castings, through the anus.
Vertebrates have evolved more complex digestive systems to adapt to their dietary needs. Some animals have a single stomach, while others have multi-chambered stomachs. Birds have developed a digestive system adapted to eating unmasticated food.
As the word monogastric suggests, this type of digestive system consists of one (“mono”) stomach chamber (“gastric”). Humans and many animals have a monogastric digestive system as illustrated in [link] ab . The process of digestion begins with the mouth and the intake of food. The teeth play an important role in masticating (chewing) or physically breaking down food into smaller particles. The enzymes present in saliva also begin to chemically break down food. The esophagus is a long tube that connects the mouth to the stomach. Using peristalsis, or wave-like smooth muscle contractions, the muscles of the esophagus push the food towards the stomach. In order to speed up the actions of enzymes in the stomach, the stomach is an extremely acidic environment, with a pH between 1.5 and 2.5. The gastric juices, which include enzymes in the stomach, act on the food particles and continue the process of digestion. Further breakdown of food takes place in the small intestine where enzymes produced by the liver, the small intestine, and the pancreas continue the process of digestion. The nutrients are absorbed into the blood stream across the epithelial cells lining the walls of the small intestines. The waste material travels on to the large intestine where water is absorbed and the drier waste material is compacted into feces; it is stored until it is excreted through the rectum.

Notification Switch
Would you like to follow the 'Biology' conversation and receive update notifications?