| << Chapter < Page | Chapter >> Page > |
Watch this Discovery Channel video on thermoregulation to see illustrations of this process in a variety of animals.
Animals can be divided into two groups: some maintain a constant body temperature in the face of differing environmental temperatures, while others have a body temperature that is the same as their environment and thus varies with the environment. Animals that do not control their body temperature are ectotherms. This group has been called cold-blooded, but the term may not apply to an animal in the desert with a very warm body temperature. In contrast to ectotherms, which rely on external temperatures to set their body temperatures, poikilotherms are animals with constantly varying internal temperatures. An animal that maintains a constant body temperature in the face of environmental changes is called a homeotherm. Endotherms are animals that rely on internal sources for body temperature but which can exhibit extremes in temperature. These animals are able to maintain a level of activity at cooler temperature, which an ectotherm cannot due to differing enzyme levels of activity.
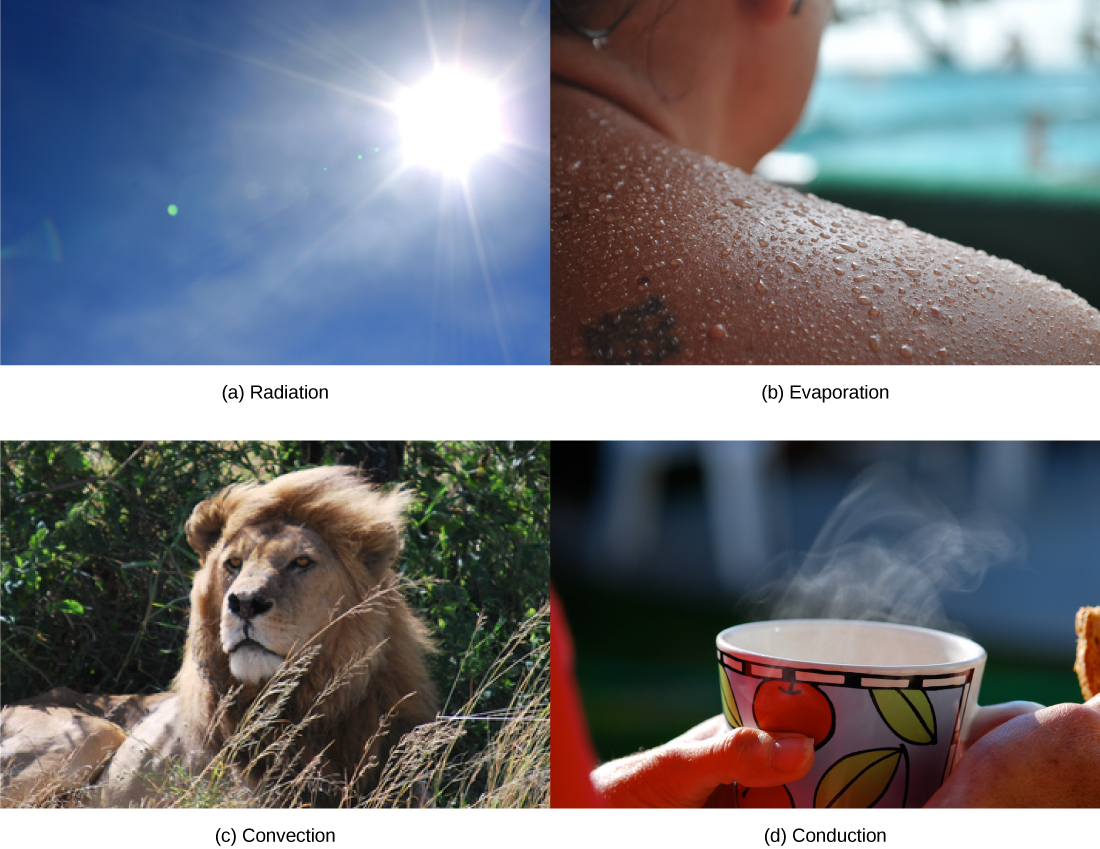
Heat can be exchanged between an animal and its environment through four mechanisms: radiation, evaporation, convection, and conduction ( [link] ). Radiation is the emission of electromagnetic “heat” waves. Heat comes from the sun in this manner and radiates from dry skin the same way. Heat can be removed with liquid from a surface during evaporation. This occurs when a mammal sweats. Convection currents of air remove heat from the surface of dry skin as the air passes over it. Heat will be conducted from one surface to another during direct contact with the surfaces, such as an animal resting on a warm rock.
Animals conserve or dissipate heat in a variety of ways. In certain climates, endothermic animals have some form of insulation, such as fur, fat, feathers, or some combination thereof. Animals with thick fur or feathers create an insulating layer of air between their skin and internal organs. Polar bears and seals live and swim in a subfreezing environment and yet maintain a constant, warm, body temperature. The arctic fox, for example, uses its fluffy tail as extra insulation when it curls up to sleep in cold weather. Mammals have a residual effect from shivering and increased muscle activity: arrector pili muscles cause “goose bumps,” causing small hairs to stand up when the individual is cold; this has the intended effect of increasing body temperature. Mammals use layers of fat to achieve the same end. Loss of significant amounts of body fat will compromise an individual’s ability to conserve heat.
Endotherms use their circulatory systems to help maintain body temperature. Vasodilation brings more blood and heat to the body surface, facilitating radiation and evaporative heat loss, which helps to cool the body. Vasoconstriction reduces blood flow in peripheral blood vessels, forcing blood toward the core and the vital organs found there, and conserving heat. Some animals have adaptions to their circulatory system that enable them to transfer heat from arteries to veins, warming blood returning to the heart. This is called a countercurrent heat exchange; it prevents the cold venous blood from cooling the heart and other internal organs. This adaption can be shut down in some animals to prevent overheating the internal organs. The countercurrent adaption is found in many animals, including dolphins, sharks, bony fish, bees, and hummingbirds. In contrast, similar adaptations can help cool endotherms when needed, such as dolphin flukes and elephant ears.

Notification Switch
Would you like to follow the 'Biology' conversation and receive update notifications?