| << Chapter < Page | Chapter >> Page > |
From 541 to 750, an outbreak of what was likely a bubonic plague (the Plague of Justinian), eliminated one-quarter to one-half of the human population in the eastern Mediterranean region. The population in Europe dropped by 50 percent during this outbreak. Bubonic plague would strike Europe more than once.
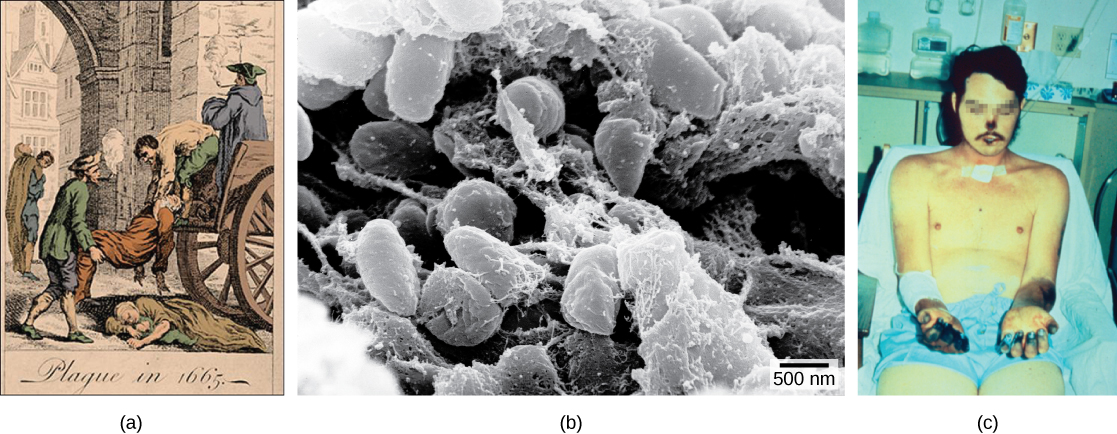
One of the most devastating pandemics was the Black Death (1346 to 1361) that is believed to have been another outbreak of bubonic plague caused by the bacterium Yersinia pestis . It is thought to have originated initially in China and spread along the Silk Road, a network of land and sea trade routes, to the Mediterranean region and Europe, carried by rat fleas living on black rats that were always present on ships. The Black Death reduced the world’s population from an estimated 450 million to about 350 to 375 million. Bubonic plague struck London hard again in the mid-1600s ( [link] ). In modern times, approximately 1,000 to 3,000 cases of plague arise globally each year. Although contracting bubonic plague before antibiotics meant almost certain death, the bacterium responds to several types of modern antibiotics, and mortality rates from plague are now very low.
Watch a video on the modern understanding of the Black Death—bubonic plague in Europe during the 14 th century.
Over the centuries, Europeans tended to develop genetic immunity to endemic infectious diseases, but when European conquerors reached the western hemisphere, they brought with them disease-causing bacteria and viruses, which triggered epidemics that completely devastated populations of Native Americans, who had no natural resistance to many European diseases. It has been estimated that up to 90 percent of Native Americans died from infectious diseases after the arrival of Europeans, making conquest of the New World a foregone conclusion.
The distribution of a particular disease is dynamic. Therefore, changes in the environment, the pathogen, or the host population can dramatically impact the spread of a disease. According to the World Health Organization (WHO) an emerging disease ( [link] ) is one that has appeared in a population for the first time, or that may have existed previously but is rapidly increasing in incidence or geographic range. This definition also includes re-emerging diseases that were previously under control. Approximately 75 percent of recently emerging infectious diseases affecting humans are zoonotic diseases, zoonoses , diseases that primarily infect animals and are transmitted to humans; some are of viral origin and some are of bacterial origin. Brucellosis is an example of a prokaryotic zoonosis that is re-emerging in some regions, and necrotizing fasciitis (commonly known as flesh-eating bacteria) has been increasing in virulence for the last 80 years for unknown reasons.

Notification Switch
Would you like to follow the 'Biology' conversation and receive update notifications?