| << Chapter < Page | Chapter >> Page > |
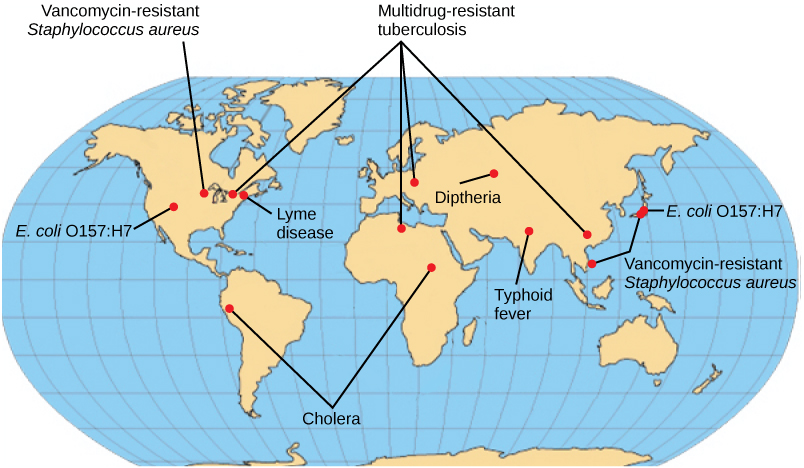
Some of the present emerging diseases are not actually new, but are diseases that were catastrophic in the past ( [link] ). They devastated populations and became dormant for a while, just to come back, sometimes more virulent than before, as was the case with bubonic plague. Other diseases, like tuberculosis, were never eradicated but were under control in some regions of the world until coming back, mostly in urban centers with high concentrations of immunocompromised people. The WHO has identified certain diseases whose worldwide re-emergence should be monitored. Among these are two viral diseases (dengue fever and yellow fever), and three bacterial diseases (diphtheria, cholera, and bubonic plague). The war against infectious diseases has no foreseeable end.
Recall that biofilms are microbial communities that are very difficult to destroy. They are responsible for diseases such as infections in patients with cystic fibrosis, Legionnaires’ disease, and otitis media. They produce dental plaque and colonize catheters, prostheses, transcutaneous and orthopedic devices, contact lenses, and internal devices such as pacemakers. They also form in open wounds and burned tissue. In healthcare environments, biofilms grow on hemodialysis machines, mechanical ventilators, shunts, and other medical equipment. In fact, 65 percent of all infections acquired in the hospital (nosocomial infections) are attributed to biofilms. Biofilms are also related to diseases contracted from food because they colonize the surfaces of vegetable leaves and meat, as well as food-processing equipment that isn’t adequately cleaned.
Biofilm infections develop gradually; sometimes, they do not cause symptoms immediately. They are rarely resolved by host defense mechanisms. Once an infection by a biofilm is established, it is very difficult to eradicate, because biofilms tend to be resistant to most of the methods used to control microbial growth, including antibiotics. Biofilms respond poorly or only temporarily to antibiotics; it has been said that they can resist up to 1,000 times the antibiotic concentrations used to kill the same bacteria when they are free-living or planktonic. An antibiotic dose that large would harm the patient; therefore, scientists are working on new ways to get rid of biofilms.

Notification Switch
Would you like to follow the 'Biology' conversation and receive update notifications?