| << Chapter < Page | Chapter >> Page > |
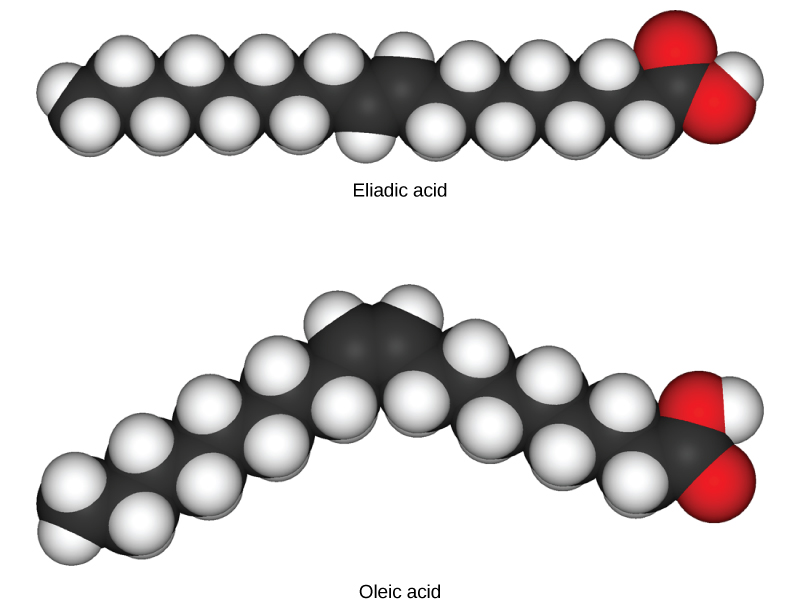
In triglycerides (fats and oils), long carbon chains known as fatty acids may contain double bonds, which can be in either the cis or trans configuration, illustrated in [link] . Fats with at least one double bond between carbon atoms are unsaturated fats. When some of these bonds are in the cis configuration, the resulting bend in the carbon backbone of the chain means that triglyceride molecules cannot pack tightly, so they remain liquid (oil) at room temperature. On the other hand, triglycerides with trans double bonds (popularly called trans fats), have relatively linear fatty acids that are able to pack tightly together at room temperature and form solid fats. In the human diet, trans fats are linked to an increased risk of cardiovascular disease, so many food manufacturers have reduced or eliminated their use in recent years. In contrast to unsaturated fats, triglycerides without double bonds between carbon atoms are called saturated fats, meaning that they contain all the hydrogen atoms available. Saturated fats are a solid at room temperature and usually of animal origin.
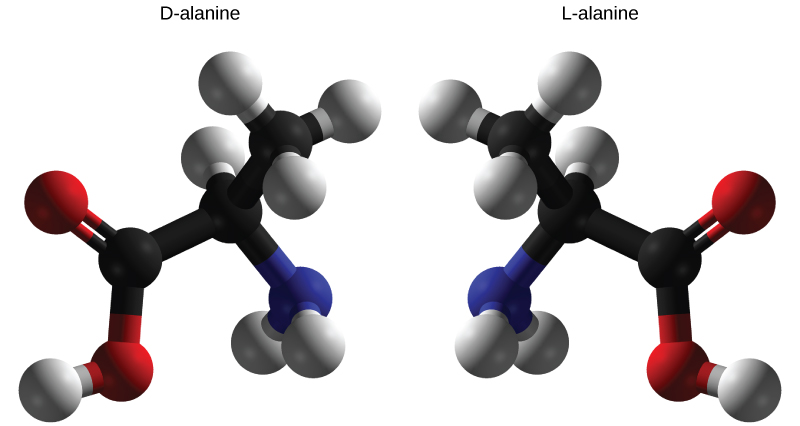
Enantiomers are molecules that share the same chemical structure and chemical bonds but differ in the three-dimensional placement of atoms so that they are mirror images. As shown in [link] , an amino acid alanine example, the two structures are non-superimposable. In nature, only the L-forms of amino acids are used to make proteins. Some D forms of amino acids are seen in the cell walls of bacteria, but never in their proteins. Similarly, the D-form of glucose is the main product of photosynthesis and the L-form of the molecule is rarely seen in nature.
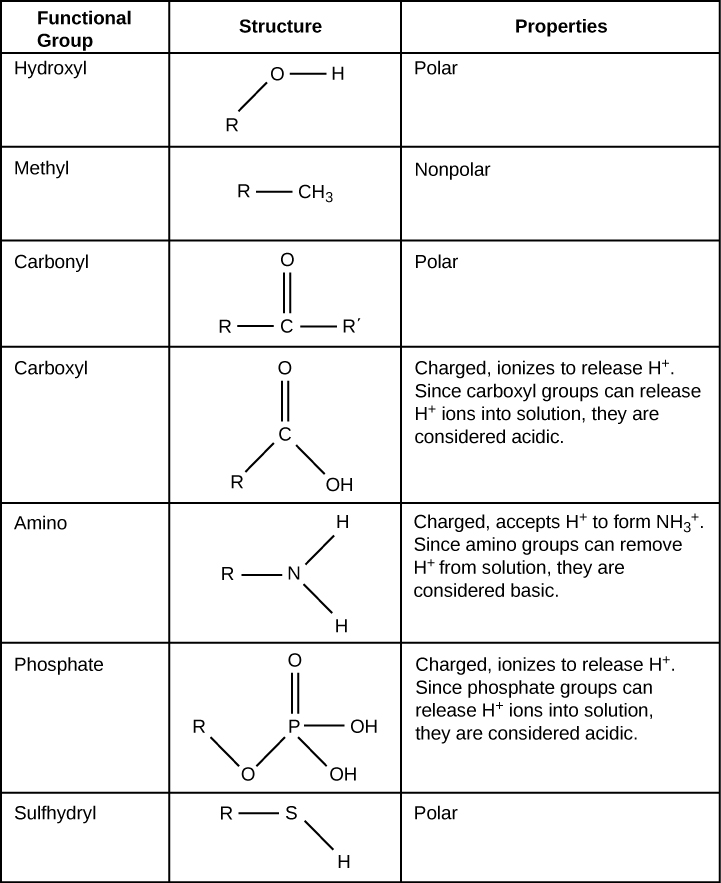
Functional groups are groups of atoms that occur within molecules and confer specific chemical properties to those molecules. They are found along the “carbon backbone” of macromolecules. This carbon backbone is formed by chains and/or rings of carbon atoms with the occasional substitution of an element such as nitrogen or oxygen. Molecules with other elements in their carbon backbone are substituted hydrocarbons .
The functional groups in a macromolecule are usually attached to the carbon backbone at one or several different places along its chain and/or ring structure. Each of the four types of macromolecules—proteins, lipids, carbohydrates, and nucleic acids—has its own characteristic set of functional groups that contributes greatly to its differing chemical properties and its function in living organisms.
A functional group can participate in specific chemical reactions. Some of the important functional groups in biological molecules are shown in [link] ; they include: hydroxyl, methyl, carbonyl, carboxyl, amino, phosphate, and sulfhydryl. These groups play an important role in the formation of molecules like DNA, proteins, carbohydrates, and lipids. Functional groups are usually classified as hydrophobic or hydrophilic depending on their charge or polarity characteristics. An example of a hydrophobic group is the non-polar methane molecule. Among the hydrophilic functional groups is the carboxyl group found in amino acids, some amino acid side chains, and the fatty acids that form triglycerides and phospholipids. This carboxyl group ionizes to release hydrogen ions (H + ) from the COOH group resulting in the negatively charged COO - group; this contributes to the hydrophilic nature of whatever molecule it is found on. Other functional groups, such as the carbonyl group, have a partially negatively charged oxygen atom that may form hydrogen bonds with water molecules, again making the molecule more hydrophilic.
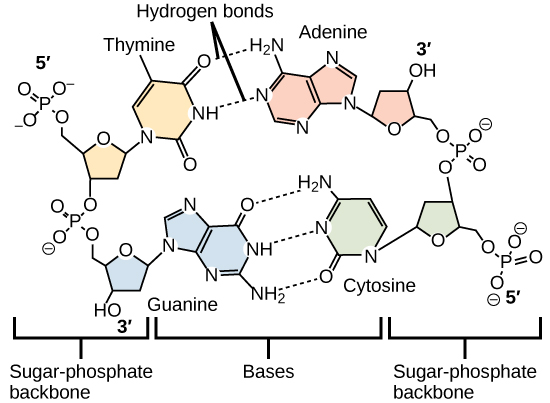
Hydrogen bonds between functional groups (within the same molecule or between different molecules) are important to the function of many macromolecules and help them to fold properly into and maintain the appropriate shape for functioning. Hydrogen bonds are also involved in various recognition processes, such as DNA complementary base pairing and the binding of an enzyme to its substrate, as illustrated in [link] .
The unique properties of carbon make it a central part of biological molecules. Carbon binds to oxygen, hydrogen, and nitrogen covalently to form the many molecules important for cellular function. Carbon has four electrons in its outermost shell and can form four bonds. Carbon and hydrogen can form hydrocarbon chains or rings. Functional groups are groups of atoms that confer specific properties to hydrocarbon (or substituted hydrocarbon) chains or rings that define their overall chemical characteristics and function.
[link] Which of the following statements is false?
[link] C

Notification Switch
Would you like to follow the 'Biology' conversation and receive update notifications?