| << Chapter < Page | Chapter >> Page > |
After an adaptive defense is produced against a pathogen, typically plasma cells first secrete IgM into the blood. BCRs on naïve B cells are of the IgM class and occasionally IgD class. IgM molecules make up approximately ten percent of all antibodies. Prior to antibody secretion, plasma cells assemble IgM molecules into pentamers (five individual antibodies) linked by a joining (J) chain, as shown in [link] . The pentamer arrangement means that these macromolecules can bind ten identical antigens. However, IgM molecules released early in the adaptive immune response do not bind to antigens as stably as IgGs, which are one of the possible types of antibodies secreted in large quantities upon re-exposure to the same pathogen. [link] summarizes the properties of immunoglobulins and illustrates their basic structures.
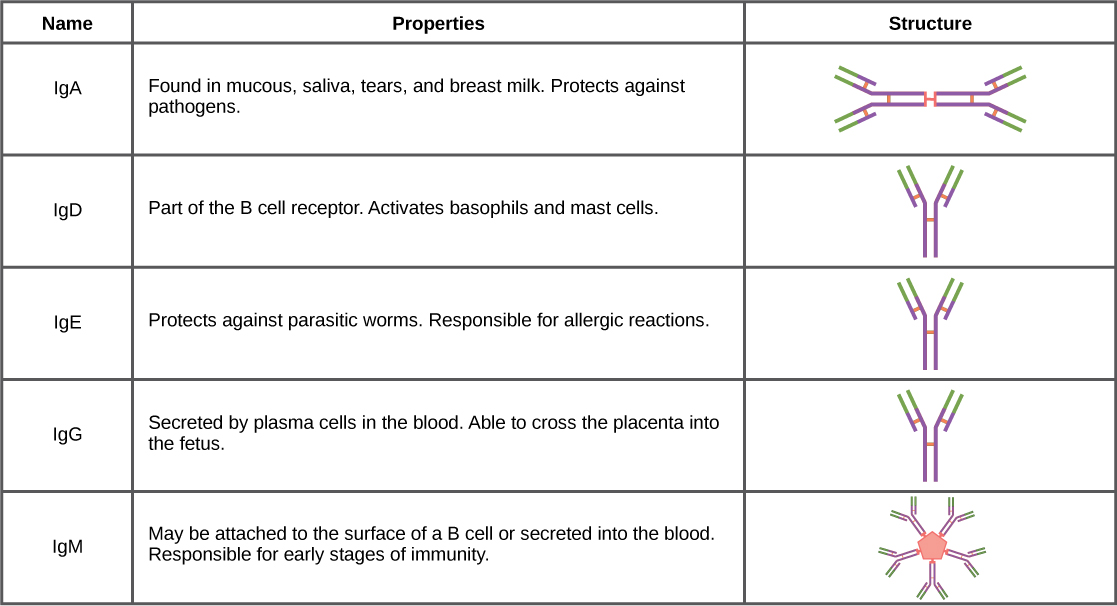
IgAs populate the saliva, tears, breast milk, and mucus secretions of the gastrointestinal, respiratory, and genitourinary tracts. Collectively, these bodily fluids coat and protect the extensive mucosa (4000 square feet in humans). The total number of IgA molecules in these bodily secretions is greater than the number of IgG molecules in the blood serum. A small amount of IgA is also secreted into the serum in monomeric form. Conversely, some IgM is secreted into bodily fluids of the mucosa. Similar to IgM, IgA molecules are secreted as polymeric structures linked with a J chain. However, IgAs are secreted mostly as dimeric molecules, not pentamers.
IgE is present in the serum in small quantities and is best characterized in its role as an allergy mediator. IgD is also present in small quantities. Similar to IgM, BCRs of the IgD class are found on the surface of naïve B cells. This class supports antigen recognition and maturation of B cells to plasma cells.
Differentiated plasma cells are crucial players in the humoral response, and the antibodies they secrete are particularly significant against extracellular pathogens and toxins. Antibodies circulate freely and act independently of plasma cells. Antibodies can be transferred from one individual to another to temporarily protect against infectious disease. For instance, a person who has recently produced a successful immune response against a particular disease agent can donate blood to a nonimmune recipient and confer temporary immunity through antibodies in the donor’s blood serum. This phenomenon is called passive immunity ; it also occurs naturally during breastfeeding, which makes breastfed infants highly resistant to infections during the first few months of life.
Antibodies coat extracellular pathogens and neutralize them, as illustrated in [link] , by blocking key sites on the pathogen that enhance their infectivity (such as receptors that “dock” pathogens on host cells). Antibody neutralization can prevent pathogens from entering and infecting host cells, as opposed to the CTL-mediated approach of killing cells that are already infected to prevent progression of an established infection. The neutralized antibody-coated pathogens can then be filtered by the spleen and eliminated in urine or feces.

Notification Switch
Would you like to follow the 'Biology' conversation and receive update notifications?