| << Chapter < Page | Chapter >> Page > |
While these and similar observations proved there was interstellar gas , they could not yet detect hydrogen, the most common element, due to its lack of spectral features in the visible part of the spectrum. (The Balmer line of hydrogen is in the visible range, but only excited hydrogen atoms produce it. In the cold interstellar medium, the hydrogen atoms are all in the ground state and no electrons are in the higher-energy levels required to produce either emission or absorption lines in the Balmer series.) Direct detection of hydrogen had to await the development of telescopes capable of seeing very-low-energy changes in hydrogen atoms in other parts of the spectrum. The first such observations were made using radio telescopes, and radio emission and absorption by interstellar hydrogen remains one of our main tools for studying the vast amounts of cold hydrogen in the universe to this day.
In 1944, while he was still a student, the Dutch astronomer Hendrik van de Hulst predicted that hydrogen would produce a strong line at a wavelength of 21 centimeters. That’s quite a long wavelength, implying that the wave has such a low frequency and low energy that it cannot come from electrons jumping between energy levels (as we discussed in Radiation and Spectra ). Instead, energy is emitted when the electron does a flip, something like an acrobat in a circus flipping upright after standing on his head.
The flip works like this: a hydrogen atom consists of a proton and an electron bound together. Both the proton and the electron act is if they were spinning like tops, and spin axes of the two tops can either be pointed in the same direction (aligned) or in opposite directions (anti-aligned). If the proton and electron were spinning in opposite directions, the atom as a whole would have a very slightly lower energy than if the two spins were aligned ( [link] ). If an atom in the lower-energy state (spins opposed) acquired a small amount of energy, then the spins of the proton and electron could be aligned, leaving the atom in a slightly excited state . If the atom then lost that same amount of energy again, it would return to its ground state. The amount of energy involved corresponds to a wave with a wavelength of 21 centimeters; hence, it is known as the 21-centimeter line .
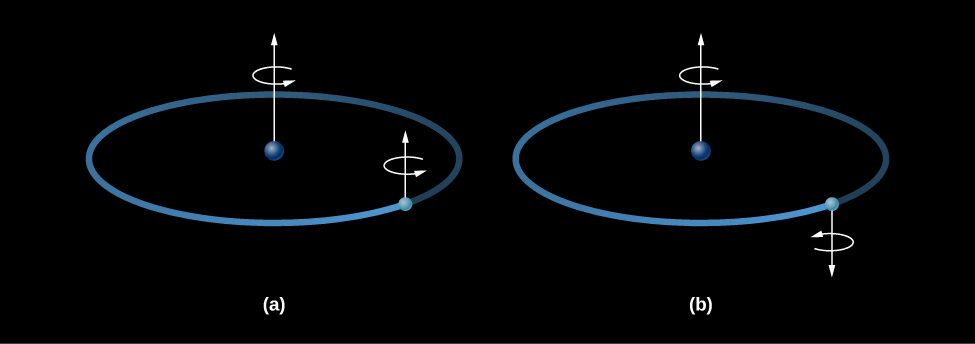
Neutral hydrogen atoms can acquire small amounts of energy through collisions with other hydrogen atoms or with free electrons. Such collisions are extremely rare in the sparse gases of interstellar space. An individual atom may wait centuries before such an encounter aligns the spins of its proton and electron. Nevertheless, over many millions of years, a significant fraction of the hydrogen atoms are excited by a collision. (Out there in cold space, that’s about as much excitement as an atom typically experiences.)

Notification Switch
Would you like to follow the 'Astronomy' conversation and receive update notifications?