| << Chapter < Page | Chapter >> Page > |
Freely available amino acids are used to create proteins. If amino acids exist in excess, the body has no capacity or mechanism for their storage; thus, they are converted into glucose or ketones, or they are decomposed. Amino acid decomposition results in hydrocarbons and nitrogenous waste. However, high concentrations of nitrogen are toxic. The urea cycle processes nitrogen and facilitates its excretion from the body.
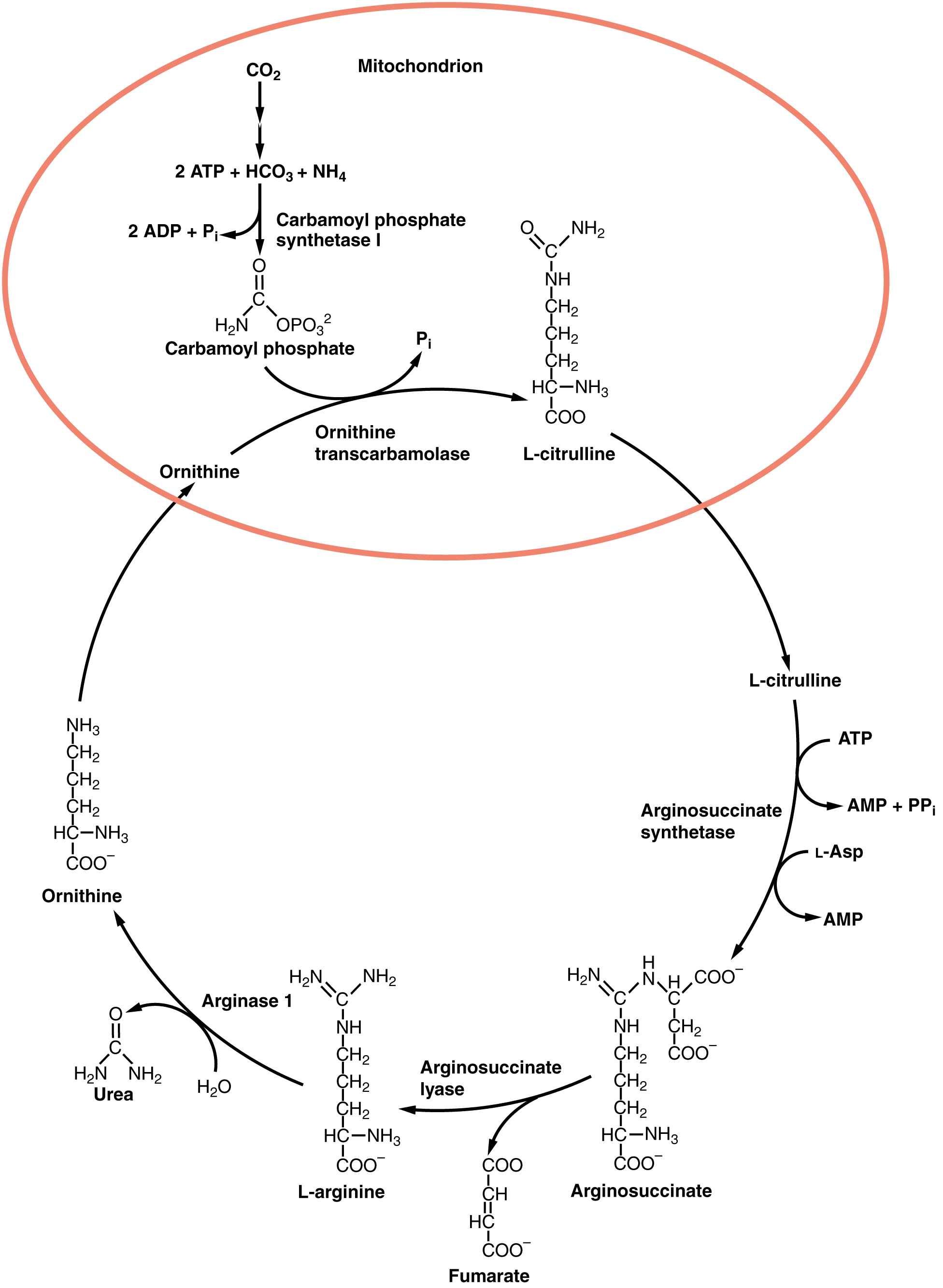
The urea cycle is a set of biochemical reactions that produces urea from ammonium ions in order to prevent a toxic level of ammonium in the body. It occurs primarily in the liver and, to a lesser extent, in the kidney. Prior to the urea cycle, ammonium ions are produced from the breakdown of amino acids. In these reactions, an amine group, or ammonium ion, from the amino acid is exchanged with a keto group on another molecule. This transamination event creates a molecule that is necessary for the Krebs cycle and an ammonium ion that enters into the urea cycle to be eliminated.
In the urea cycle, ammonium is combined with CO 2 , resulting in urea and water. The urea is eliminated through the kidneys in the urine ( [link] ).
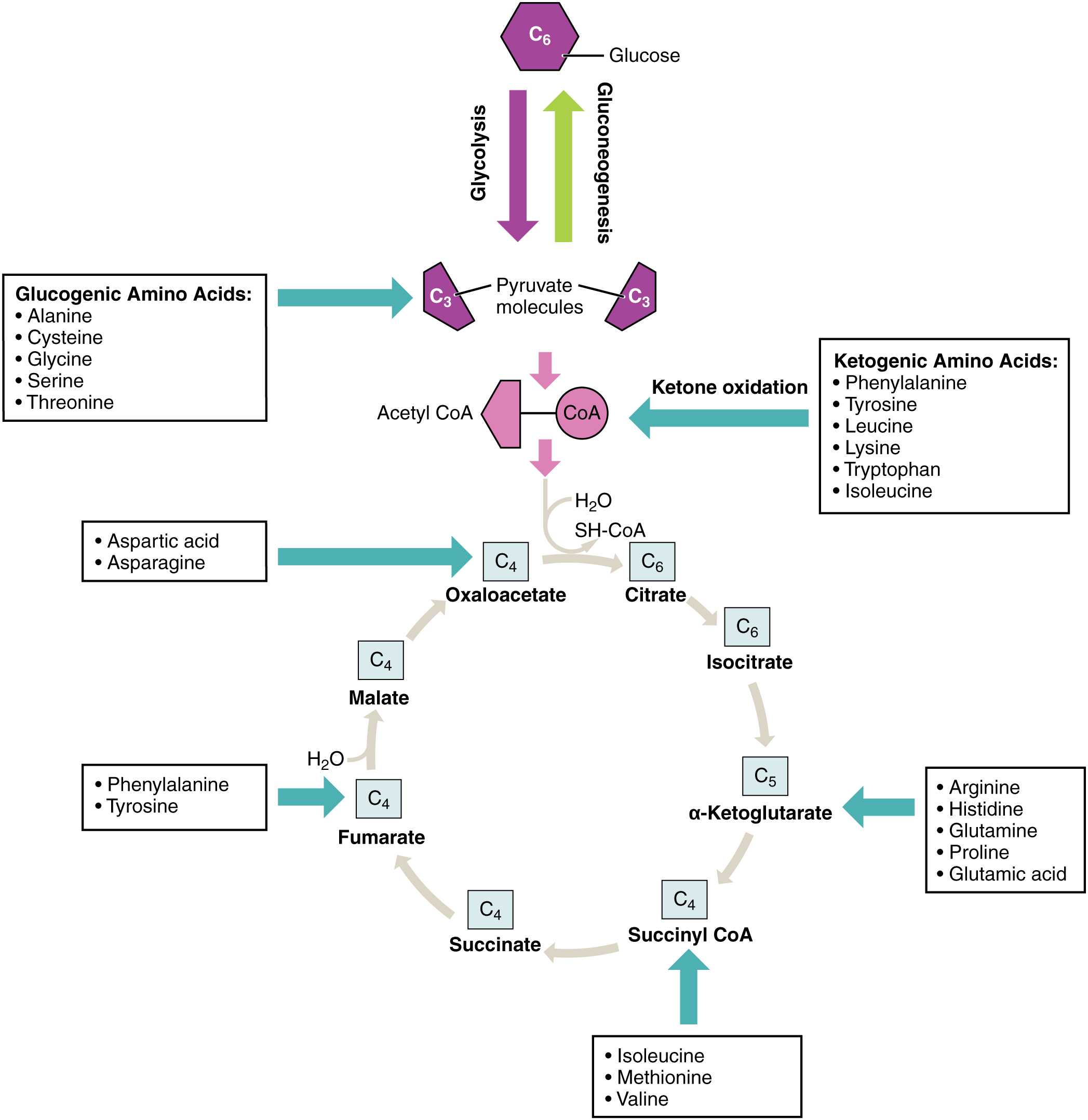
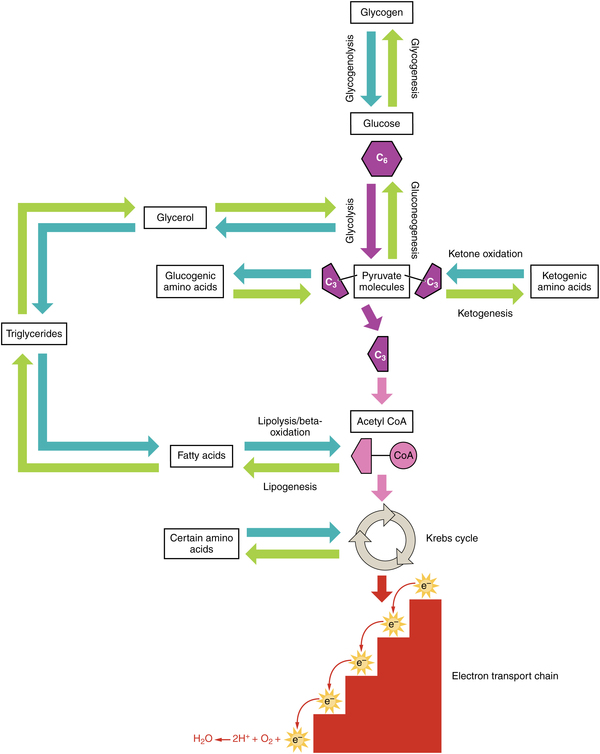
Amino acids can also be used as a source of energy, especially in times of starvation. Because the processing of amino acids results in the creation of metabolic intermediates, including pyruvate, acetyl CoA, acetoacyl CoA, oxaloacetate, and α-ketoglutarate, amino acids can serve as a source of energy production through the Krebs cycle ( [link] ). [link] summarizes the pathways of catabolism and anabolism for carbohydrates, lipids, and proteins.
PKU affects about 1 in every 15,000 births in the United States. People afflicted with PKU lack sufficient activity of the enzyme phenylalanine hydroxylase and are therefore unable to break down phenylalanine into tyrosine adequately. Because of this, levels of phenylalanine rise to toxic levels in the body, which results in damage to the central nervous system and brain. Symptoms include delayed neurological development, hyperactivity, mental retardation, seizures, skin rash, tremors, and uncontrolled movements of the arms and legs. Pregnant women with PKU are at a high risk for exposing the fetus to too much phenylalanine, which can cross the placenta and affect fetal development. Babies exposed to excess phenylalanine in utero may present with heart defects, physical and/or mental retardation, and microcephaly. Every infant in the United States and Canada is tested at birth to determine whether PKU is present. The earlier a modified diet is begun, the less severe the symptoms will be. The person must closely follow a strict diet that is low in phenylalanine to avoid symptoms and damage. Phenylalanine is found in high concentrations in artificial sweeteners, including aspartame. Therefore, these sweeteners must be avoided. Some animal products and certain starches are also high in phenylalanine, and intake of these foods should be carefully monitored.
Digestion of proteins begins in the stomach, where HCl and pepsin begin the process of breaking down proteins into their constituent amino acids. As the chyme enters the small intestine, it mixes with bicarbonate and digestive enzymes. The bicarbonate neutralizes the acidic HCl, and the digestive enzymes break down the proteins into smaller peptides and amino acids. Digestive hormones secretin and CCK are released from the small intestine to aid in digestive processes, and digestive proenzymes are released from the pancreas (trypsinogen and chymotrypsinogen). Enterokinase, an enzyme located in the wall of the small intestine, activates trypsin, which in turn activates chymotrypsin. These enzymes liberate the individual amino acids that are then transported via sodium-amino acid transporters across the intestinal wall into the cell. The amino acids are then transported into the bloodstream for dispersal to the liver and cells throughout the body to be used to create new proteins. When in excess, the amino acids are processed and stored as glucose or ketones. The nitrogen waste that is liberated in this process is converted to urea in the urea acid cycle and eliminated in the urine. In times of starvation, amino acids can be used as an energy source and processed through the Krebs cycle.

Notification Switch
Would you like to follow the 'Anatomy & Physiology' conversation and receive update notifications?