| << Chapter < Page | Chapter >> Page > |
Simplify each of the following quotients as much as possible using the power of a quotient rule. Write answers with positive exponents.
Recall that to simplify an expression means to rewrite it by combing terms or exponents; in other words, to write the expression more simply with fewer terms. The rules for exponents may be combined to simplify expressions.
Simplify each expression and write the answer with positive exponents only.
Simplify each expression and write the answer with positive exponents only.
Recall at the beginning of the section that we found the number when describing bits of information in digital images. Other extreme numbers include the width of a human hair, which is about 0.00005 m, and the radius of an electron, which is about 0.00000000000047 m. How can we effectively work read, compare, and calculate with numbers such as these?
A shorthand method of writing very small and very large numbers is called scientific notation , in which we express numbers in terms of exponents of 10. To write a number in scientific notation, move the decimal point to the right of the first digit in the number. Write the digits as a decimal number between 1 and 10. Count the number of places n that you moved the decimal point. Multiply the decimal number by 10 raised to a power of n . If you moved the decimal left as in a very large number, is positive. If you moved the decimal right as in a small large number, is negative.
For example, consider the number 2,780,418. Move the decimal left until it is to the right of the first nonzero digit, which is 2.
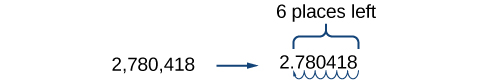
We obtain 2.780418 by moving the decimal point 6 places to the left. Therefore, the exponent of 10 is 6, and it is positive because we moved the decimal point to the left. This is what we should expect for a large number.
Working with small numbers is similar. Take, for example, the radius of an electron, 0.00000000000047 m. Perform the same series of steps as above, except move the decimal point to the right.

Notification Switch
Would you like to follow the 'College algebra' conversation and receive update notifications?