| << Chapter < Page | Chapter >> Page > |
As mentioned before, quantification of TOC in the dry quantification method will proceed via complete combustion of the sample in a carbon free atmosphere (typically a pure oxygen atmosphere). Quantification of sample is performed via non-dispersive infrared detection cell. A characteristic asymmetric stretching at 2350 cm -1 can be seen for CO 2 . The intensity of this infrared signal CO 2 is proportional to the quantity of CO 2 in the sample. Therefore, in order to translate signal intensity to amount, a calibration curve is constructed from known amounts of pure calcium carbonate, looking specifically at the intensity of the CO 2 peak. One may point out that calcium carbonate is an inorganic source of carbon, but it is important to note that the source of carbon has no effect on its quantification. Preparation of a calibration curve follows similar preparation as to an analyte, while no pre-treatment with acid is needed, the standards must be thoroughly dried in an oven. When a sample is ready to be analyzed, it is first weighed on some form of analytical balance, and then placed in the combustion analyzer, such as a LECO analyzer, where the oven and the non-dispersive IR cell are one machine ( [link] ).

Combustion proceeds at temperatures in the excess of 1350 o C in a stream of pure oxygen. Comparing the intensity of your characteristic IR peak to the intensities of the characteristic IR peaks of your known standards, the TOC of the sample can be determined. By comparing the mass of the sample to the mass of carbon obtained from the analyzer, the % organic carbon in the sample can be determined according to [link] .
Use of this dry technique is most common for rock and other solid samples. In the oil and gas industry, it is extremely important to know the organic carbon content of rock samples in order to ascertain production viability of a well. The sample can be loaded in the LECO combustion analyzer and pyrolyzed in order to quantify TOC.
As shown in [link] the total carbon in a sample (TC) is the sum of the inorganic forms of carbon and organic forms of carbon in a sample.
It is known that no other sources of carbon contribute to the TC determination because no other sources of carbon exist. So in theory, if one could quantify the TOC by a method described in the previous section, and follow that with a measurement of the TIC in the pre-treatment acid waste, one could find the TC of a sample by summing the value obtained for TIC and the value obtained for TOC. However, in TC quantification this is hardly done: partly in order to avoid propagation of error associated with the other two methods, also cost restraints.
In measuring TC of a sample, the same dry technique of combustion of the sample is used, just like in the quantification of TOC. The same analyzer used to measure TOC can handle a TC measurement. No sample pre-treatment with acid is needed, so it is important to remember that the characteristic peak of CO 2 now seen is representative of the carbon of the entire sample. Now using [link] , the TIC carbon of the sample can be found as well. Subtraction of the TOC from the measured TC in the analyzer gives the value for TIC.
Direct methods to measure the TIC of a sample, in addition to indirect measurement by taking advantage of [link] , are possible. Typical TIC measurements are done on water samples, where the alkalinity and hardness of water is a result of inorganic carbonates, be it bicarbonate or carbonate. Treatment of these types of samples follows similar procedures to treatment of samples for organic carbon. A sample of water is acidified, such that the equilibrium, [link] obeys Le Chatelier’s principle and favors the release of CO 2 . The CO 2 released can be measured in a variety of different ways
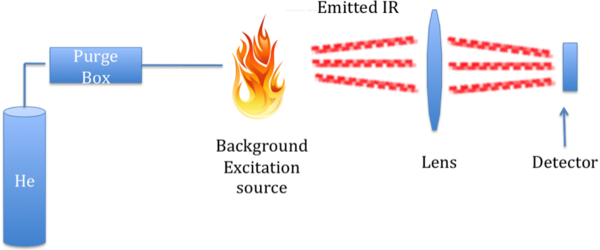
As with the combustion technique for measuring TC and TOC, measurement of the intensity of the characteristic IR stretch for CO 2 compared to standards can be used to quantity of TIC in a sample. However, in this case, it is emission of IR radiation that is measured, not absorption. An instrument that can do such a measurement is a FIRE-TIC, meaning Flame IR emission. This instrument consists of a purge like devices connected to a FIRE detector, as shown in [link] .
Measurement of Carbon content is crucial for a lot of industries. In this module you have seen a variety of ways to measure Total Carbon TC, as well as the source of that carbon, whether it be organic in nature (TOC), or inorganic (TIC). This information is extremely important for several industries: from oil exploration, where information on carbon content is needed to evaluate a formation’s production viability, to regulatory agencies, where carbon content and its origin are needed to ensure quality control and public safety.
TOC, TC, TIC measurements do have significant limitations. Mostly all techniques are destructive in nature, meaning that sample cannot be recovered. Further limitations include assumptions that have to be made in the measurement. In TOC measurement for example, assumptions that all TIC has been removed in pretreatments with acid have to be made, as well as that all organic carbon is completely oxidized to CO 2 . In TIC measurements, it is assumed that all carbon sources are removed from the sample and detected. Several things can be done to promote these conditions so as to make such assumptions valid.
All measurements cost money, because TOC, TIC, and TC are all related by [link] , more frequently than not only two measurements are done, and the third value is found by using their relation to one another.

Notification Switch
Would you like to follow the 'Physical methods in chemistry and nano science' conversation and receive update notifications?