| << Chapter < Page | Chapter >> Page > |
For example, in the reaction [link] , the stoichiometry is determined as shown in [link] and [link] .
However, as calculated this reaction would be for the reaction in an environment of pure oxygen. On the other hand, air has only 21% oxygen (78% nitrogen, 1% noble gases). Therefore, if air is used as the oxidizer, this must be taken into account in the calculations, i.e., [link] .
The mole fractions for a stoichiometric mixture in air are therefore calculated in following way: [link] - [link] ).

Calculate the fuel mole fraction (xfuel) for the stoichiometric reaction:
In this reaction ν = 2, as 2 moles of oxygen are needed to fully oxidize methane into H 2 O and CO 2 .
Calculate the fuel mole fraction for the stoichiometric reaction
The fuel mole fraction is 4.03%.
Premixed combustion reactions can also be characterized by the air equivalence ratio, λ, as shown in [link] .
The fuel equivalence ratio, Φ, is the reciprocal of this value [link] .
Rewriting [link] in terms of the fuel equivalence ratio gives: [link] - [link] .

The premixed combustion processes can also be identified by their air and fuel equivalence ratios ( [link] ).
| Type of combustion | Φ | λ |
|---|---|---|
| Rich | >1 | <1 |
| Stoichiometric | =1 | =1 |
| Lean | <1 | >1 |
With a premixed type of combustion, there is much greater control over the reaction. If performed at lean conditions, then high temperatures, the pollutant nitric oxide, and the production of soot can be minimized or even avoided, allowing the system to combust efficiently. However, a premixed system requires large volumes of premixed reactants, which pose a fire hazard. As a result, nonpremixed combusted, while not being efficient, is more commonly used.
Though the instrumentation of combustion analysis has greatly improved, the basic components of the apparatus ( [link] ) have not changed much since the late 18th century.

The sample of an organic compound, such as a hydrocarbon, is contained within a furnace or exposed to a flame and burned in the presence of oxygen, creating water vapor and carbon dioxide gas ( [link] ). The sample moves first through the apparatus to a chamber in which H 2 O is absorbed by a hydrophilic substance and second through a chamber in which CO 2 is absorbed. The change in weight of each chamber is determined to calculate the weight of H 2 O and CO 2 . After the masses of H 2 O and CO 2 have been determined, they can be used to characterize and calculate the composition of the original sample.
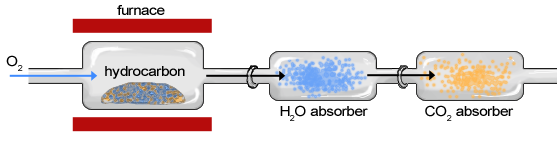
Combustion analysis is a standard method of determining a chemical formula of a substance that contains hydrogen and carbon. First, a sample is weighed and then burned in a furnace in the presence of excess oxygen. All of the carbon is converted to carbon dioxide, and the hydrogen is converted to water in this way. Each of these are absorbed in separate compartments, which are weighed before and after the reaction. From these measurements, the chemical formula can be determined.

Notification Switch
Would you like to follow the 'Physical methods in chemistry and nano science' conversation and receive update notifications?