| << Chapter < Page | Chapter >> Page > |
An atom inside a crystal of any material will have a coordination number (n) determined by the structure of the material. For example, all atoms within the bulk of a silicon crystal will be in a tetrahedral four-coordinate environment (n = 4). However, at the surface of a crystal the atoms will not make their full compliment of bonds. Each atom will therefore have less nearest neighbors than an atom within the bulk of the material. The missing bonds are commonly called dangling bonds. While this description is not particularly accurate it is, however, widely employed and as such will be used herein. The number of dangling bonds may be defined as the difference between the ideal coordination number (determined by the bulk crystal structure) and the actual coordination number as observed at the surface.
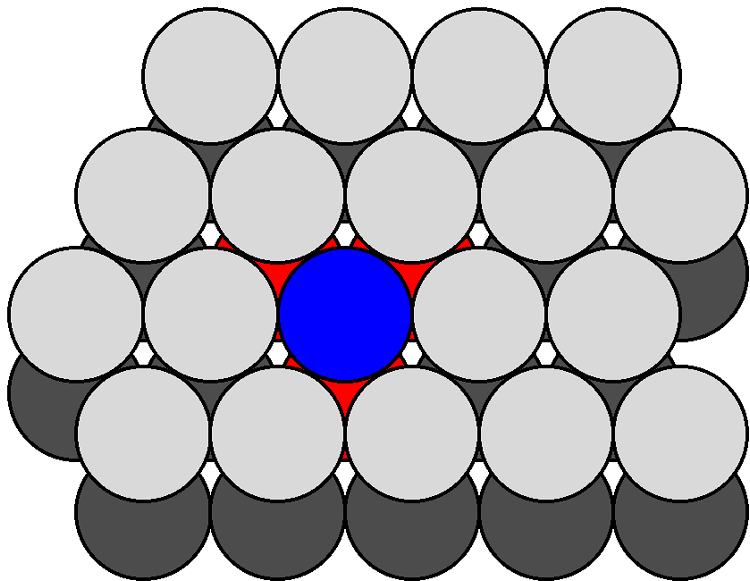
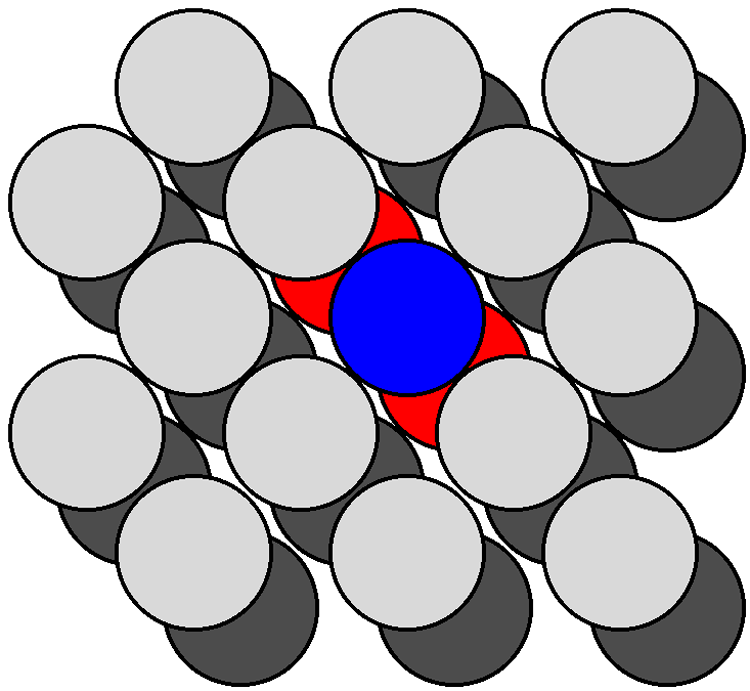
[link] shows a section of the {111} surfaces of a diamond cubic lattice viewed perpendicular to the {111} plane. The atoms within the bulk have a coordination number of four. In contrast, the atoms at the surface (e.g., the atom shown in blue in [link] ) are each bonded to just three other atoms (the atoms shown in red in [link] ), thus each surface atom has one dangling bond. As can be seen from [link] , which shows the atoms at the {100} surface viewed perpendicular to the {100} plane, each atom at the surface (e.g., the atom shown in blue in [link] ) is only coordinated to two other atoms (the atoms shown in red in [link] ), leaving two dangling bonds per atom. It should be noted that the same number of dangling bonds are found for the {111} and {100} planes of a zinc blende lattice. The ratio of dangling bonds for the {100} and {111} planes of all diamond cubic and zinc blende structures is {100}:{111} = 2:1. Furthermore, since the atom densities of each plane are known then the ratio of the dangling bond densities is determined to be: {100}:{111} = 1:0.577.
For silicon, the {111} planes are closer packed than the {100} planes. As a result, growth of a silicon crystal is therefore slowest in the<111>direction, since it requires laying down a close packed atomic layer upon another layer in its closest packed form. As a consequence<111>Si is the easiest to grow, and therefore the least expensive.
The dissolution or etching of a crystal is related to the number of broken bonds already present at the surface: the fewer bonds to be broken in order to remove an individual atom from a crystal, the easier it will be to dissolve the crystal. As a consequence of having only one dangling bond (requiring three bonds to be broken) etching silicon is slowest in the<111>direction. The electronic properties of a silicon wafer are also related to the number of dangling bonds.
Silicon microcircuits are generally formed on a single crystal wafer that is diced after fabrication by either sawing part way through the wafer thickness or scoring (scribing) the surface, and then physically breaking. The physical breakage of the wafer occurs along the natural cleavage planes, which in the case of silicon are the {111} planes.
The zinc blende lattice observed for gallium arsenide results in additional considerations over that of silicon. Although the {100} plane of GaAs is structurally similar to that of silicon, two possibilities exist: a face consisting of either all gallium atoms or all arsenic atoms. In either case the surface atoms have two dangling bonds, and the properties of the face are independent of whether the face is gallium or arsenic.
The {111} plane also has the possibility of consisting of all gallium or all arsenic. However, unlike the {100} planes there is a significant difference between the two possibilities. [link] shows the gallium arsenide structure represented by two interpenetrating fcc lattices. The [111] axis is vertical within the plane of the page. Although the structure consists of alternate layers of gallium and arsenic stacked along the [111]axis, the distance between the successive layers alternates between large and small. Assigning arsenic as the parent lattice the order of the layers in the [111] direction is As-Ga-As-Ga-As-Ga, while in the direction the layers are ordered, Ga-As-Ga-As-Ga-As ( [link] ). In silicon these two directions are of course identical. The surface of a crystal would be either arsenic, with three dangling bonds, or gallium, with one dangling bond. Clearly, the latter is energetically more favorable. Thus, the (111) plane shown in [link] is called the (111) Ga face. Conversely, the plane would be either gallium, with three dangling bonds, or arsenic, with one dangling bond. Again, the latter is energetically more favorable and the plane is therefore called the (111) As face.
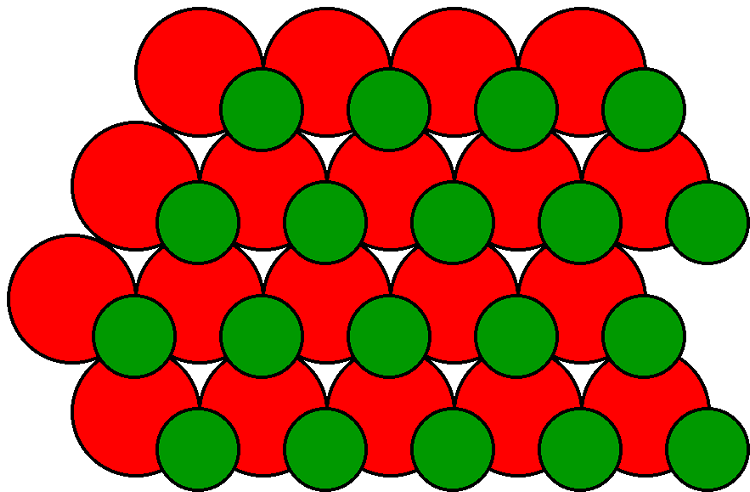
The (111) As is distinct from that of (111) Ga due to the difference in the number of electrons at the surface. As a consequence, the (111) As face etches more rapidly than the (111) Ga face. In addition, surface evaporation below 770 °C occurs more rapidly at the (111) As face.

Notification Switch
Would you like to follow the 'Physical methods in chemistry and nano science' conversation and receive update notifications?