| << Chapter < Page | Chapter >> Page > |
In a larger hydrocarbon, the structural formula of the molecule is generally not predictable from the number of carbon atoms and the number of hydrogen atoms, because there may be more than one possible arrangement. In these cases, the molecular structure must be given to deduce the Lewis structure and thus the arrangement of the electrons in the molecule. However, with this information, it is straightforward to create a Lewis structure for molecules with the general molecular formula C n H 2n+2 such as propane, butane, etc. For example, the Lewis structure for “normal” butane (with all carbons linked one after another) is found to be:
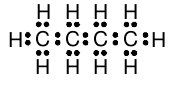
There are no hydrocarbons where the number of hydrogen atoms is greater than two more than twice the number of carbons. For example, CH 5 does not exist, nor does C 2 H 8 . If we try to draw Lewis structures for CH 5 or C 2 H 8 which are consistent with the octet rule, we will find that there is no way to do so. Similarly, CH 3 and C 2 H 5 are observed to be so extremely reactive that it is impossible to prepare stable quantities of either compound. And again we will find that it is not possible to draw Lewis structures for these molecules which obey the octet rule.
We come to a very important and powerful conclusion: when it is possible to draw a Lewis structure in which each carbon has a complete octet of electrons in its valence shell, the corresponding molecule will be stable and the hydrocarbon compound will exist under ordinary conditions. After working a few examples, it is apparent that this always holds for compounds with molecular formula C n H 2n+2 .
Although our model so far does a good job of describing molecules with the formula C n H 2n+2 , there are many stable hydrocarbon compounds with molecular formulae in which the number of hydrogen atoms is less than 2n+2. Simple examples are ethene C 2 H 4 and acetylene C 2 H 2 in which there are not enough hydrogen atoms to permit each carbon atom to be bonded to four atoms each. In each molecule, the two carbon atoms must be bonded to one another. When we arrange the electrons so that the carbon atoms share a single pair of electrons and then attach hydrogen atoms to each carbon, we wind up with rather unsatisfying Lewis structures for ethene and acetylene:
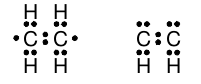
Note that, in these structures, neither carbon atom has a complete octet of valence shell electrons, but these are both stable compounds. We need to extend our model to work for these types of molecules.
These structures indicate that the carbon-carbon bonds in ethane, ethene, and acetylene should be very similar, since in each case a single pair of electrons is shared by the two carbons. However, we can observe that the carbon-carbon bonds in these molecules are very different chemically and physically. First, we can compare the energy required to break each bond (the “bond energy” or “bond strength”). Second, it is possible to observe the distance between the two carbon atoms, which is referred to as the “bond length.” Bond lengths are typically measured in picometers (1 picometer (pm) = 10 -12 m).

Notification Switch
Would you like to follow the 'Concept development studies in chemistry 2012' conversation and receive update notifications?