| << Chapter < Page | Chapter >> Page > |
Returning to the reactions used to introduce this topic, they may now both be identified as redox processes. In the reaction between sodium and chlorine to yield sodium chloride, sodium is oxidized (its oxidation number increases from 0 in Na to +1 in NaCl) and chlorine is reduced (its oxidation number decreases from 0 in Cl 2 to –1 in NaCl). In the reaction between molecular hydrogen and chlorine, hydrogen is oxidized (its oxidation number increases from 0 in H 2 to +1 in HCl) and chlorine is reduced (its oxidation number decreases from 0 in Cl 2 to –1 in HCl).
Several subclasses of redox reactions are recognized, including combustion reactions in which the reductant (also called a fuel ) and oxidant (often, but not necessarily, molecular oxygen) react vigorously and produce significant amounts of heat, and often light, in the form of a flame. Solid rocket-fuel reactions such as the one depicted in [link] are combustion processes. A typical propellant reaction in which solid aluminum is oxidized by ammonium perchlorate is represented by this equation:

Watch a brief video showing the test firing of a small-scale, prototype, hybrid rocket engine planned for use in the new Space Launch System being developed by NASA. The first engines firing at 3 s (green flame) use a liquid fuel/oxidant mixture, and the second, more powerful engines firing at 4 s (yellow flame) use a solid mixture.
Single-displacement (replacement) reactions are redox reactions in which an ion in solution is displaced (or replaced) via the oxidation of a metallic element. One common example of this type of reaction is the acid oxidation of certain metals:
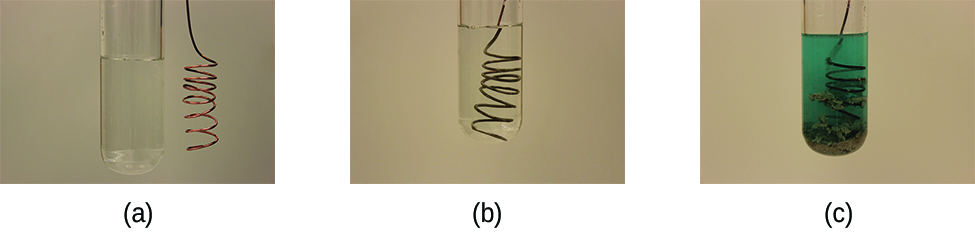
Metallic elements may also be oxidized by solutions of other metal salts; for example:
This reaction may be observed by placing copper wire in a solution containing a dissolved silver salt. Silver ions in solution are reduced to elemental silver at the surface of the copper wire, and the resulting Cu 2+ ions dissolve in the solution to yield a characteristic blue color ( [link] ).
(a)
(b)
(c)
(d)
(e)
(a) This is not a redox reaction, since oxidation numbers remain unchanged for all elements.
(b) This is a redox reaction. Gallium is oxidized, its oxidation number increasing from 0 in Ga( l ) to +3 in GaBr 3 ( s ). The reducing agent is Ga( l ). Bromine is reduced, its oxidation number decreasing from 0 in Br 2 ( l ) to –1 in GaBr 3 ( s ). The oxidizing agent is Br 2 ( l ).
(c) This is a redox reaction. It is a particularly interesting process, as it involves the same element, oxygen, undergoing both oxidation and reduction (a so-called disproportionation reaction) . Oxygen is oxidized, its oxidation number increasing from –1 in H 2 O 2 ( aq ) to 0 in O 2 ( g ). Oxygen is also reduced, its oxidation number decreasing from –1 in H 2 O 2 ( aq ) to –2 in H 2 O( l ). For disproportionation reactions, the same substance functions as an oxidant and a reductant.
(d) This is not a redox reaction, since oxidation numbers remain unchanged for all elements.
(e) This is a redox reaction (combustion). Carbon is oxidized, its oxidation number increasing from –2 in C 2 H 4 ( g ) to +4 in CO 2 ( g ). The reducing agent (fuel) is C 2 H 4 ( g ). Oxygen is reduced, its oxidation number decreasing from 0 in O 2 ( g ) to –2 in H 2 O( l ). The oxidizing agent is O 2 ( g ).
Is this a redox reaction? If so, provide a more specific name for the reaction if appropriate, and identify the oxidant and reductant.
Yes, a single-replacement reaction. Sn( s ) is the reductant, HCl( g ) is the oxidant.

Notification Switch
Would you like to follow the 'Ucd bis2a intro to biology v1.2' conversation and receive update notifications?