| << Chapter < Page | Chapter >> Page > |
So, lets look at the reactions in Figure 1 from a reduction potential perspective. In figure 2, we look at the same reaction and half reactions, but now we are looking at them through the lens of reduction potentials:
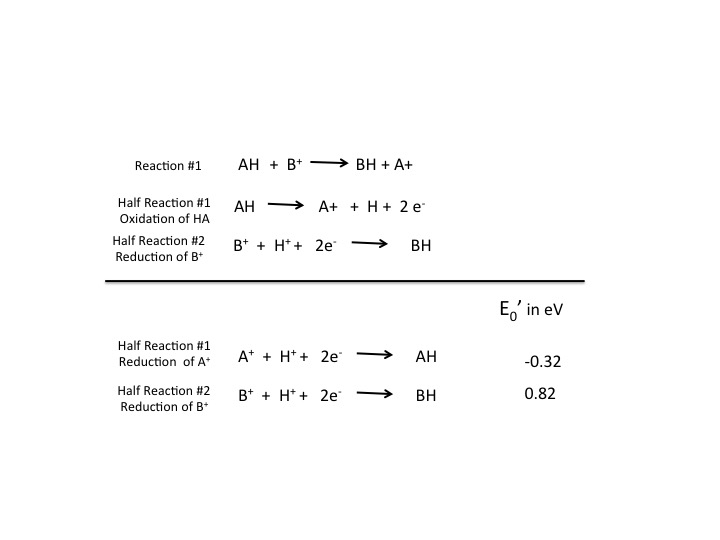
In the reaction written above (figure 2) the E' values are given for the two half reactions. Which of the following statements is accurate when comparing two half reactions?
g
In figure 2 the top half of the figure shows the two half reactions as described in the original reaction. If we look at the substrates, HA and B + , in the reaction, HA is being oxidize A + and B + is being reduced. If we rearrange the equations a bit, at least half reaction #1, such that we look at it from a reduction perspective we get the new half reaction shown in the bottom half of the figure. Now we are looking at the oxidized form of the molecule as the substrates and the reduced form as the products.
Once all reactions are written in the form for a reduction potential, it is easy to see who will take the electrons, the compound with the more positive reduction potential. In our generic case, its B + . This nomenclature will be useful as we will see when we discuss and use the electron tower a tool to aid in understanding electron movements in biological systems.
The question now becomes how much potential energy the can cell derive from each red/ox reaction? The answer lies in the change of the reduction potentials of the two compounds. The change in the reduction potential for the reaction or E 0 ' for the reaction is the difference between the E 0 ' for the oxidant (the compound getting the electrons and causing the oxidation of the other compound) and the reductant (the compound losing the electrons and causing the reduction of the other compound). In our generic example in Figures 1 and 2 AH is the reductant and B + is the oxidant, remember electrons are flowing from HA to B + . Using the E 0 ' of -0.32 for the reductant and 0.82 for the oxidant the total change in E 0 ' or ΔE 0 ' is 1.14 eV. The change in reduction potential is readily convertible to changes in Gibbs free energy, ΔG , for the reaction as we will see below. In other words the change in reduction potential has a corresponding value in the change in free energy. While you do not need to know the details of how to convert from free energy to reduction potential and visa verse, suffice it to say, there is a correlation. In general a large positive ΔE 0 ' is proportional to a large negative ΔG. The reactions are exergonic and spontaneous.

Notification Switch
Would you like to follow the 'Ucd bis2a intro to biology v1.2' conversation and receive update notifications?