| << Chapter < Page | Chapter >> Page > |
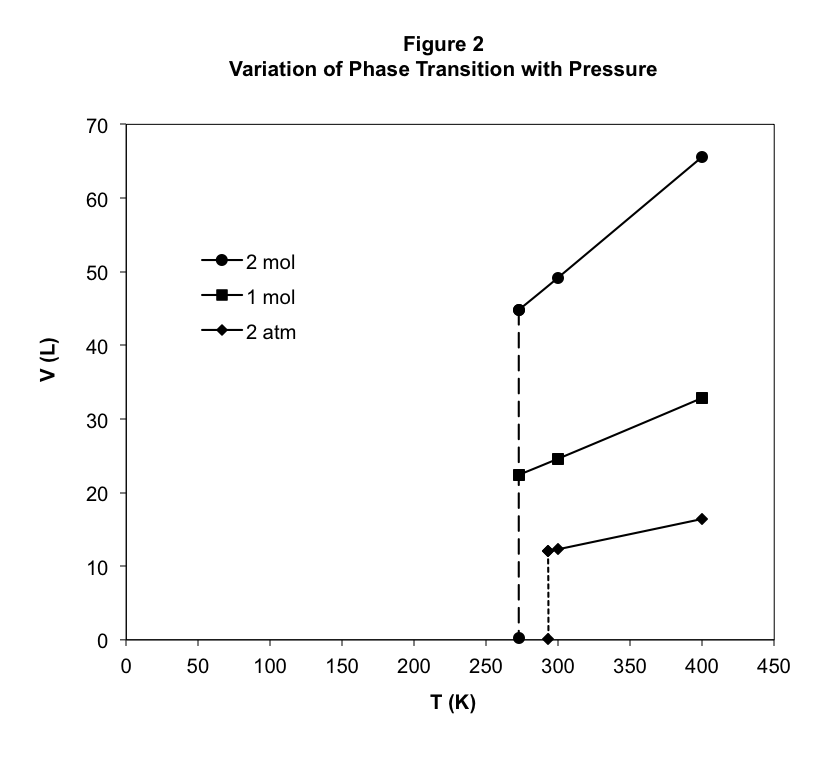
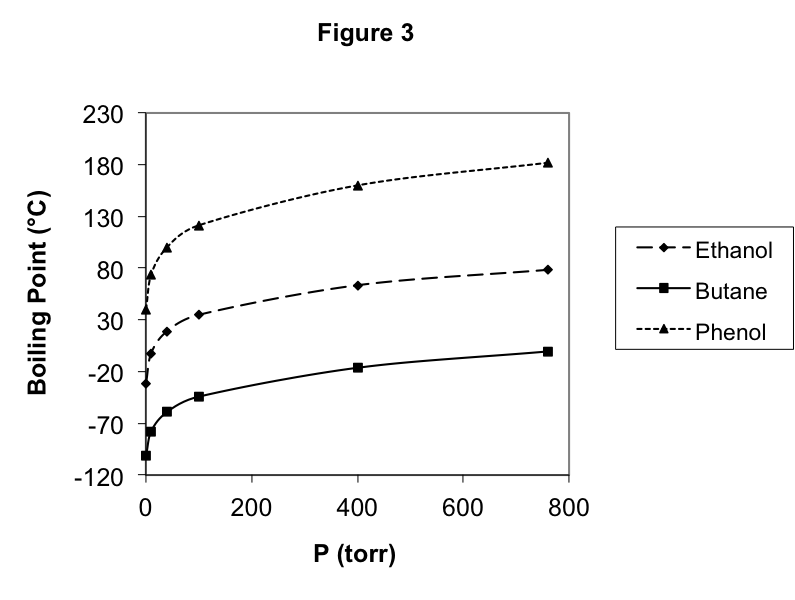
Next, let’s see what happens when we vary the applied pressure by cooling 1.00 mol of butane at a constant 2.00 atm pressure, instead of 1.00 atm. The result is also shown in Figure 2 as the lowest line. We can see the now familiar phase transition with a similar dramatic drop in volume. However, in this case, we find that the phase transition occurs at 293.2 K, over 20 K higher than at the lower pressure. Clearly, the temperature of the phase transition depends on the applied pressure. We can measure the boiling point temperature of butane for many values of the applied pressure, and these results are plotted in Figure 3.
Finally, we consider varying the substance trapped in the cylinder, replacing the butane with ethanol or phenol. We discover that the boiling point temperature depends on both the substance identity as well as on the applied pressure. As we found before, the boiling point does not depend on the amount of the substance we trap. In Figure 3, we have also plotted the boiling point as a function of the applied pressure for these three substances. It is very clear that the boiling points for different substances can be very different from one another, although the variation of the boiling point with pressure looks similar from one substance to the next.
Our previous observations indicate that, for a given pressure, there is a phase transition temperature for liquid and gas: below the boiling point, the liquid is the only “stable” phase which exists, and any gas that might exist at that point will spontaneously condense into liquid. Above the boiling point, the gas is the only stable phase and any liquid present will spontaneously evaporate.
However, it is a common observation that any liquid left in an open container will, under most conditions, eventually evaporate, even if the temperature of the liquid is well below the normal boiling point. As a simple example, puddles of water on sidewalks evaporate at air temperatures far below the 100 ºC boiling point of water. This everyday observation only seems surprising in light of the discussion above. Why would liquid water spontaneously evaporate if liquid is the more stable phase below the boiling point? We clearly have more work to do to understand phase transitions, including evaporation.
The tendency of a liquid to evaporate is referred to as its “volatility”: a more volatile liquid evaporates more readily. We can make a quantitative measure of liquid volatility. We slightly modify our previous cylinder-piston apparatus by adding a gauge to measure the pressure of gas inside the cylinder. (See Figure 4 for an illustration.) We begin with liquid water only in the cylinder with an applied pressure of 1 atm at a temperature of 25 ºC, well below its boiling point. We now pull back the piston by an arbitrary amount, and then we lock the piston in place, fixing the volume trapped inside the cylinder. Since we have now opened up a large cavity in the space above the liquid water, we might expect that the pressure inside the cylinder to decrease, creating a vacuum in that space. If so, we would expect that the pressure inside the cylinder is small or zero.

Notification Switch
Would you like to follow the 'Concept development studies in chemistry 2013' conversation and receive update notifications?