| << Chapter < Page | Chapter >> Page > |
For the energy of chemical reactions, what standard shall we pick for comparing energies? One way is to pick the set of substances from which we can form all possible other materials. These are, of course, the elements. How would this work? Let’s take a specific example. Butene, C 4 H 8 , reacts with hydrogen to form butane, C 4 H 10 :
To find the heat of this reaction ∆H, we need to compare the energy of the products to the energy of the reactants. We could do this by subtracting the energy of the products, relative to the energy of the elements, minus the energy of the reactants, again relative to the energy of the same elements. Clearly it would be very helpful to measure these energies.
The enthalpy of a substance, say butane, relative to the elements which make it up, carbon and hydrogen, is called the “enthalpy of formation” of the substance. This is the energy that is either absorbed or released when the substance is made from the elements in their standard form, and is labeled as ∆H f . For example, for butane, the formation reaction from the elements is
and the heat of this reaction is called the enthalpy of formation of butane. The enthalpy of formation tells us the energy of butane relative to the elements from which it could be formed.
Of course, for this to be useful, we have to measure this quantity. This formation reaction for butane, [link] , is virtually impossible to perform in a controlled way so that we can measure the energy of the reaction. But, due to the work we did in the previous study on Hess’ Law, we have a way to measure the energy of this reaction without actually carrying out the reaction. We can use an alternative pathway. Since carbon, hydrogen, and butane are all easy to burn, we can use combustion reactions as the alternative pathway and measure the energy of these reactions.
Using Hess’ Law, we can use these three reactions and energies to find the energy of the formation reaction for butane, in other words, the enthalpy of formation of butane. [link] shows a diagram of Hess’ Law for [link] from which we can write:
This gives the enthalpy of formation of butane ∆H f (C 4 H 10 ) = -125 kJ/mol.
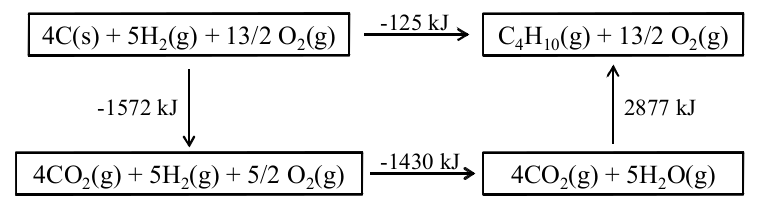
Remember that this enthalpy of formation of butane is the energy of [link] and therefore measures the energy of butane relative to the elements which make it up.
We can use this same approach to observe and measure the enthalpy of formation of any substance we are interested in. The enthalpies of formation of several interesting compounds are listed in [link] . These values allow us to compare the energies of different compounds, since these are all relative to the same standard.
| Substance | Formula | ΔH˚ f (kJ/mol) |
| Acetylene | C 2 H 2 (g) | 226.7 |
| Ammonia | NH 3 (g) | -46.1 |
| Carbon Dioxide | CO 2 (g) | -393.5 |
| Carbon Monoxide | CO (g) | -110.5 |
| Ethanol | C 2 H 5 OH (l) | -277.7 |
| Ethylene | C 2 H 4 (g) | 52.3 |
| Glucose | C 6 H 12 O 6 (s) | -1260 |
| Hydrogen Chloride | HCl (g) | -92.3 |
| Iron(III) Oxide | Fe 2 O 3 (s) | -824.2 |
| Magnesium Carbonate | MgCO 3 (s) | -1095.8 |
| Methane | CH 4 (g) | -74.8 |
| Nitric Oxide | NO (g) | 90.2 |
| Water (g) | H 2 O (g) | -241.8 |
| Water (l) | H 2 O (l) | -285.8 |

Notification Switch
Would you like to follow the 'Concept development studies in chemistry 2012' conversation and receive update notifications?