| << Chapter < Page | Chapter >> Page > |
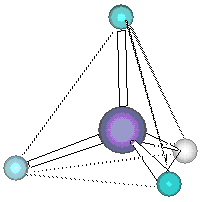
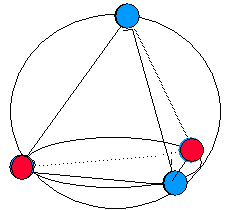
We conclude that molecular geometry is determined by minimizing the mutual repulsion of the valence shellelectron pairs. As such, this model of molecular geometry is often referred to as the valence shell electron pair repulsion (VSEPR) theory . For reasons that will become clear, extension of this model impliesthat a better name is the Electron Domain (ED) Theory .
This model also accounts, at least approximately, for the bond angles ofH 2 O andNh 3 . These molecules are clearly not tetrahedral, likeCH 4 , since neither contains the requisite five atoms to form thetetrahedron. However, each molecule does contain a central atom surrounded by four pairs of valence shell electrons. We expect fromour Electron Domain model that those four pairs should be arrayed in a tetrahedron, without regard to whether they are bonding orlone-pair electrons. Then attaching the hydrogens (two for oxygen, three for nitrogen) produces a prediction of bond angles of109.5 °, very close indeed to the observed angles of 104.5 ° inH 2 O and 107 ° inNH 3 .
Note, however, that we do not describe the geometries ofH 2 O andNH 3 as "tetrahedral," since the atoms of the molecules do not form tetrahedrons, even if the valence shell electron pairs do. (It isworth noting that these angles are not exactly equal to 109.5 °, as in methane. These deviations will be discussed later .)
We have developed the Electron Domain model to this point only for geometries of molecules with four pairs ofvalence shell electrons. However, there are a great variety of molecules in which atoms from Period 3 and beyond can have morethan an octet of valence electrons. We consider two such molecules illustrated in [link] .
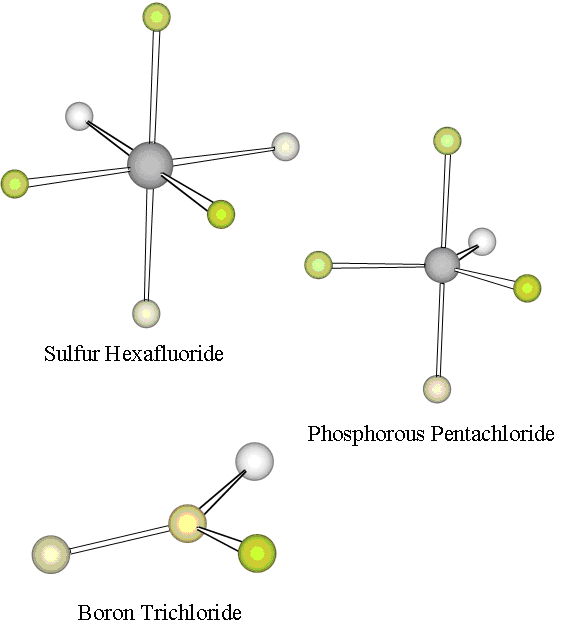
First, PCl 5 is a stable gaseous compound in which the five chlorine atoms areeach bonded to the phosphorous atom. Experiments reveal that the geometry ofPCl 5 is that of a trigonal bipyramid : three of the chlorine atoms form an equilateral triangle with the P atom in the center, and theother two chlorine atoms are on top of and below the P atom. Thus there must be 10 valence shell electrons around the phosphorousatom. Hence, phosphorous exhibits what is called an expanded valence in PCl 5 . Applying our Electron Domain model, we expect the five valenceshell electron pairs to spread out optimally to minimize their repulsions. The required geometry can again be found by trying toplace five points on the surface of a sphere with maximum distances amongst these points. A little experimentation reveals that thiscan be achieved by placing the five points to form a trigonal bipyramid. Hence, Electron Domain theory accounts for the geometryof PCl 5 .
Second, SF 6 is a fairly unreactive gaseous compound in which all six fluorineatoms are bonded to the central sulfur atom. Again, it is clear that the octet rule is violated by the sulfur atom, which musttherefore have an expanded valence. The observed geometry of SF 6 , as shown in [link] , is highly symmetric: all bond lengths are identical and all bond angles are90 °. The F atoms form an octahedron about the central S atom: four of the F atoms form a square with the S atom at the center, and the othertwo F atoms are above and below the S atom. To apply our Electron Domain model to understand this geometry, we must place six points,representing the six electron pairs about the central S atom, on the surface of a sphere with maximum distances between the points.The requisite geometry is found, in fact, to be that of an octahedron, in agreement with the observed geometry.

Notification Switch
Would you like to follow the 'Concept development studies in chemistry 2013' conversation and receive update notifications?