| << Chapter < Page | Chapter >> Page > |
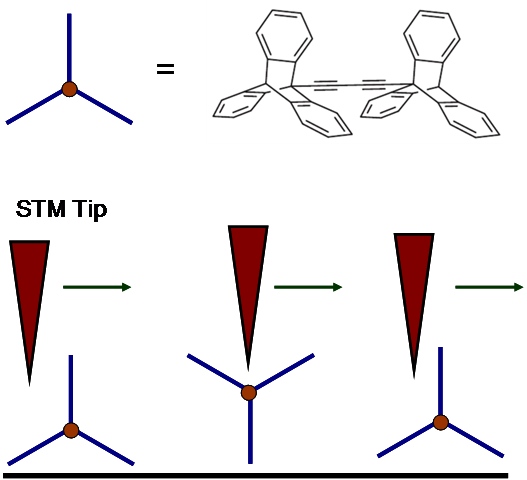
A triptycene wheeled dimeric molecule [link] was also synthesized for studying rolling motion under STM. This "tripod-like" triptycene wheel ulike a ball like C 60 molecule also demonstrated a rolling motion on the surface. The two triptycene units were connected via a dialkynyl axle, for both desired molecule orientation sitting on surface and directional preference of the rolling motion. STM controlling and imaging was demonstrated, including the mechanism [link] .
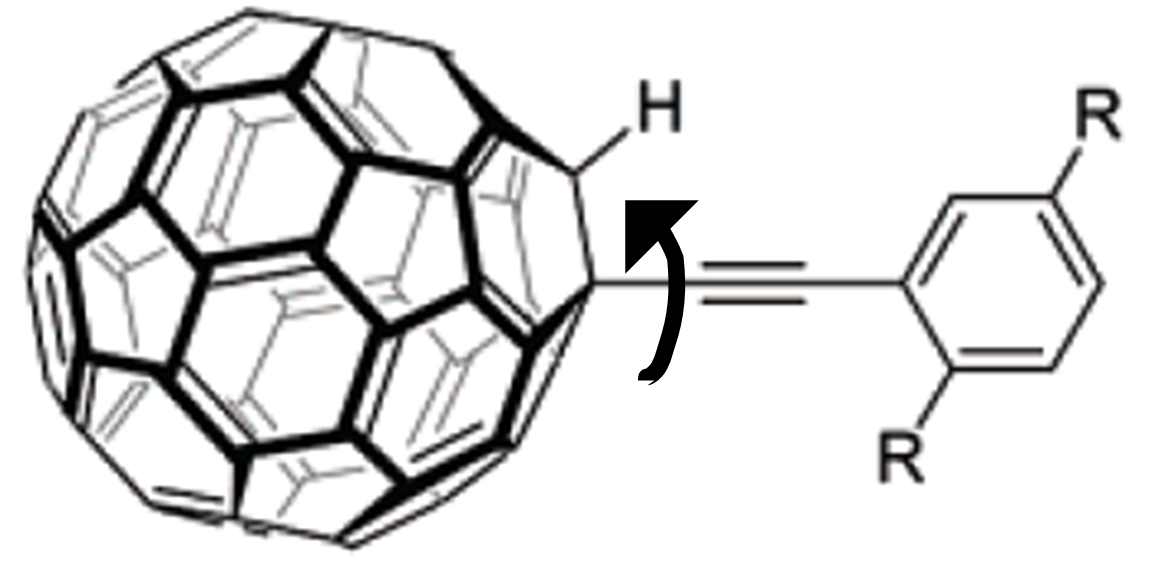
Another use of STM imaging at single molecule imaging is the single molecule nanocar by the Tour group at Rice University. The concept of a nanocar initially employed the free rotation of a C-C single bond between a spherical C 60 molecule and an alkyne, [link] . Based on this concept, an “axle” can be designed into which are mounted C 60 “wheels” connected with a “chassis” to construct the “nanocar”. Nanocars with this design are expected to have a directional movement perpendicular to the axle. Unfortunately, the first generation nanocar (named “nanotruck” [link] ) encountered some difficulties in STM imaging due to its chemical instability and insolubility. Therefore, a new of design of nanocar based on OPE has been synthesized [link] .
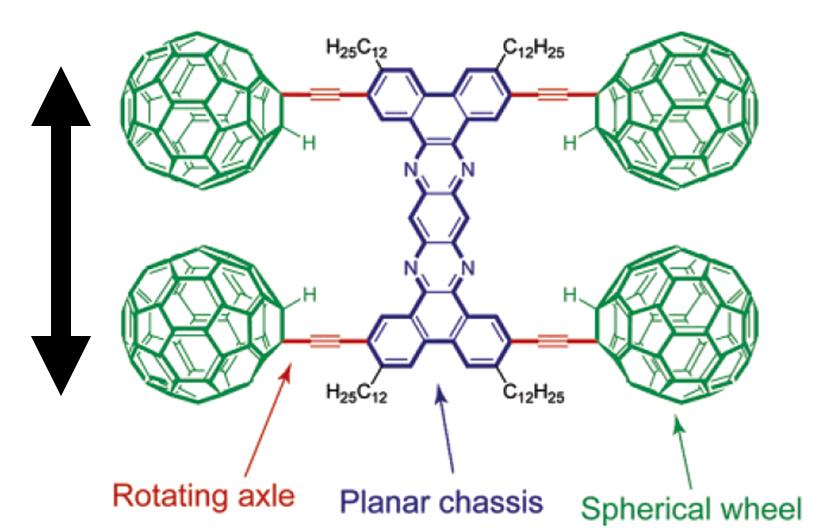
The newly designed nanocar was studied with STM. When the nanocar was heated to ~200 °C, noticeable displacements of the nanocar were observed under selected images from a 10 min STM experiment [link] . The phenomenon that the nanocar moved only at high temperature was attributed their stability to a relatively strong adhesion force between the fullerene wheels and the underlying gold. The series of images showed both pivotal and translational motions on the surfaces.
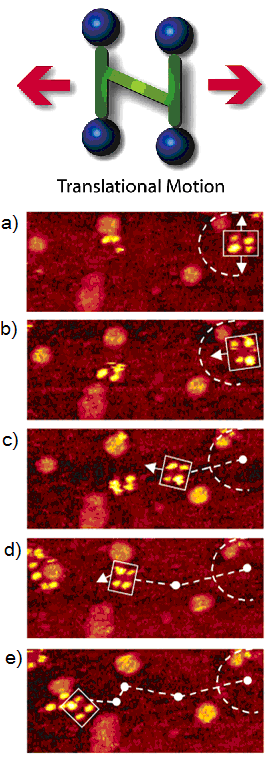
Although literature studies suggested that the C 60 molecule rolls on the surface, in the nanocar movement studies it is still not possible to conclusively conclude that the nanocar moves on surface exclusively via a rolling mechanism. Hopping, sliding and other moving modes could also be responsible for the movement of the nanocar since the experiment was carried out at high temperature conditions, making the C 60 molecules more energetic to overcome interactions between surfaces.
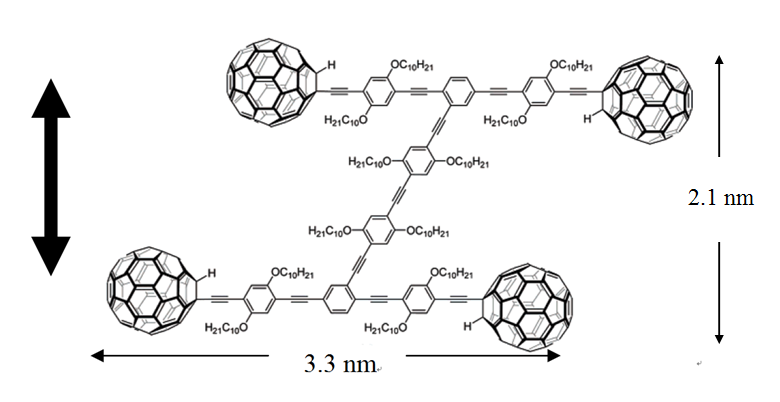
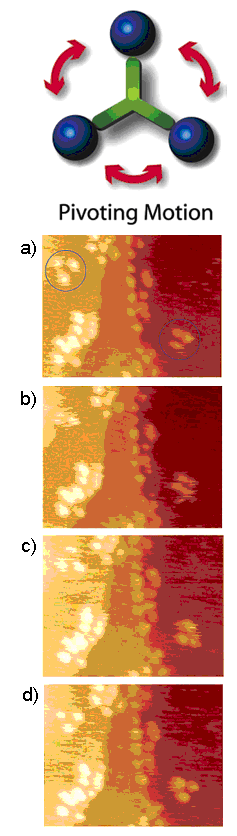
To tackle the question of the mode of translation, a trimeric “nano-tricycle” has been synthesized. If the movement of fullerene-wheeled nanocar was based on a hopping or sliding mechanism, the trimer should give observable translational motions like the four-wheeled nanocar, however, if rolling is the operable motion then the nano-tricycle should rotate on an axis, but not translate across the surface. The result of the imaging experiment of the trimer at ~200 °C ( [link] ,) yielded very small and insignificant translational displacements in comparison to 4-wheel nanocar ( [link] ). The trimeric 3-wheel nanocar showed some pivoting motions in the images. This motion type can be attributed to the directional preferences of the wheels mounted on the trimer causing the car to rotate. All the experimental results suggested that a C 60 -based nanocar moves via a rolling motion rather than hopping and sliding. In addition, the fact that the thermally driven nanocar only moves in high temperature also suggests that four C 60 have very strong interactions to the surface.

Notification Switch
Would you like to follow the 'Nanomaterials and nanotechnology' conversation and receive update notifications?