| << Chapter < Page | Chapter >> Page > |
The UV-visible absorbance spectroscopy is a characterization technique in which the absorbance of the material is studied as a function of wavelength. The visible region of the spectrum is in the wavelength range of 380 nm (violet) to 740 nm (red) and the near ultraviolet region extends to wavelengths of about 200 nm. The UV-visible spectrophotometer analyzes over the wavelength range 200 – 900 nm.
When the Group 12-16 semiconductor nanocrystals are exposed to light having an energy that matches a possible electronic transition as dictated by laws of quantum physics, the light is absorbed and an exciton pair is formed. The UV-visible spectrophotometer records the wavelength at which the absorption occurs along with the intensity of the absorption at each wavelength. This is recorded in a graph of absorbance of the nanocrystal versus wavelength.
A working schematic of the UV-visible spectrophotometer is shown in [link] .
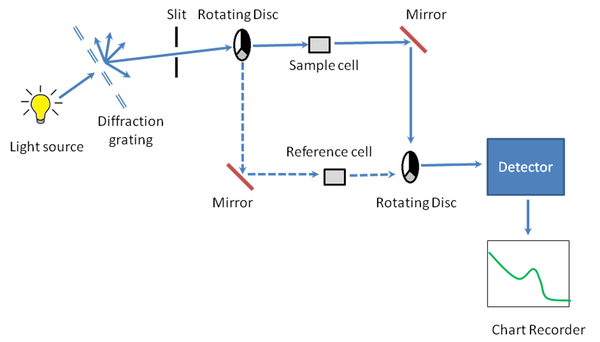
Since it is a UV-vis spectrophotometer, the light source ( [link] ) needs to cover the entire visible and the near ultra-violet region (200 - 900 nm). Since it is not possible to get this range of wavelengths from a single lamp, a combination of a deuterium lamp for the UV region of the spectrum and tungsten or halogen lamp for the visible region is used. This output is then sent through a diffraction grating as shown in the schematic.
The beam of light from the visible and/or UV light source is then separated into its component wavelengths (like a very efficient prism) by a diffraction grating ( [link] ). Following the slit is a slit that sends a monochromatic beam into the next section of the spectrophotometer.
Light from the slit then falls onto a rotating disc ( [link] ). Each disc consists of different segments – an opaque black section, a transparent section and a mirrored section. If the light hits the transparent section, it will go straight through the sample cell, get reflected by a mirror, hits the mirrored section of a second rotating disc, and then collected by the detector. Else if the light hits the mirrored section, gets reflected by a mirror, passes through the reference cell, hits the transparent section of a second rotating disc and then collected by the detector. Finally if the light hits the black opaque section, it is blocked and no light passes through the instrument, thus enabling the system to make corrections for any current generated by the detector in the absence of light.
For liquid samples, a square cross section tube sealed at one end is used. The choice of cuvette depends on the following factors:
| Cuvette | Wavelength (nm) |
| Visible only glass | 380 - 780 |
| Visible only plastic | 380 - 780 |
| UV plastic | 220 - 780 |
| Quartz | 200 - 900 |

Notification Switch
Would you like to follow the 'Nanomaterials and nanotechnology' conversation and receive update notifications?