| << Chapter < Page | Chapter >> Page > |
You have a sample that contains 5 moles of zinc.
Molar mass of zinc is 65.38 g.mol , meaning that 1 mole of zinc has a mass of 65.38 g.
If 1 mole of zinc has a mass of 65.38 g, then 5 moles of zinc has a mass of:
65.38 g x 5 mol = 326.9 g (answer to a)
(answer to b)
The calculations that have been used so far, can be made much simpler by using the following equation:
The equation can also be used to calculate mass and molar mass, using the following equations:
and
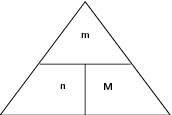
The following diagram may help to remember the relationship between these three variables. You need to imagine that the horizontal line is like a 'division' sign and that the vertical line is like a 'multiplication' sign. So, for example, if you want to calculate 'M', then the remaining two letters in the triangle are 'm' and 'n' and 'm' is above 'n' with a division sign between them. In your calculation then, 'm' will be the numerator and 'n' will be the denominator.
Calculate the number of moles of copper there are in a sample that weighs 127 g.
There are 2 moles of copper in the sample.
You are given a 5 mol sample of sodium. What mass of sodium is in the sample?
M = 22.99 g.mol
Therefore,
The sample of sodium has a mass of 114.95 g.
Calculate the number of atoms there are in a sample of aluminium that weighs 80.94 g.
Number of atoms in 3 mol aluminium = 3 6.023 10
There are 18.069 10 aluminium atoms in a sample of 80.94 g.
So far, we have only discussed moles, mass and molar mass in relation to elements . But what happens if we are dealing with a molecule or some other chemical compound? Do the same concepts and rules apply? The answer is 'yes'. However, you need to remember that all your calculations will apply to the whole molecule . So, when you calculate the molar mass of a molecule, you will need to add the molar mass of each atom in that compound. Also, the number of moles will also apply to the whole molecule. For example, if you have one mole of nitric acid (HNO ), it means you have 6.023 x molecules of nitric acid in the sample. This also means that there are 6.023 10 atoms of hydrogen, 6.023 10 atoms of nitrogen and (3 6.023 10 ) atoms of oxygen in the sample.

Notification Switch
Would you like to follow the 'Siyavula textbooks: grade 11 physical science' conversation and receive update notifications?