| << Chapter < Page | Chapter >> Page > |
A second conclusion has to do with the ion created by the acid ionization. The negative ion produced has asurplus electron, and the relative energy of this ion will depend on how readily that extra electron is attracted to the atoms ofion. The more electronegative those atoms are, the stronger is the attraction. Therefore, theOCl – ion can more readily accommodate the negative charge than can the OBr – ion. And the OClO – ion can more readily accommodate the negative charge than can the OCl – ion.
We conclude that the presence of strongly electronegative atoms in an acid increases the polarization of theH-O bond, thus facilitating ionization of the acid, and increases the attraction of the extra electron to the negative ion, thusstabilizing the negative ion. Both of these factors increase the acid strength. Chemists commonly use both of these conclusions inunderstanding and predicting relative acid strength.
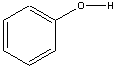
The relative acidity of carbon compounds is a major subject of organic chemistry, which we can only visit brieflyhere. In each of the carboxylic acids, the H-O group is attached to a carbonyl C=O group, which is in turn bonded to other atoms. Thecomparison we observe here is between carboxylic acid molecules, denoted asRCOOH, and other organic molecules containing the H-O group, such asalcohols denoted as ROH.(R is simply an atom or group of atoms attached to the functional group.) The former are obviously acids whereas the latter groupcontains molecules which are generally extremely weak acids. One interesting comparison is for the acid and alcohol when R is thebenzene ring,C 6 H 5 . Benzoic acid,C 6 H 5 COOH, has , whereas phenol,C 6 H 5 OH, has . Thus, the presence of the doubly bonded oxygen atom on the carbonatom adjacent to the O-H clearly increases the acidity of the molecule, and thus increases ionization of the O-H bond.
This observation is quite reasonable in the context of our previous conclusion. Adding an electronegativeoxygen atom in near proximity to the O-H bond both increases the polarization of the O-H bond and stabilizes the negative ionproduced by the acid ionization. In addition to the electronegativity effect, carboxylate anions,RCOO – , exhibit resonance stabilization, as seen in [link] .
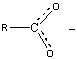
The resonance results in a sharing of the negative charge over several atoms, thus stabilizing the negativeion. This is a major contributing factor in the acidity of carboxylic acids versus alcohols.

Notification Switch
Would you like to follow the 'Ucd bis2a intro to biology v1.2' conversation and receive update notifications?