| << Chapter < Page | Chapter >> Page > |
For example, thinking back to [link] where butene reacts to become butane, it is interesting to compare the enthalpy of formation of these two compounds to see that the energy of butene is higher than the energy of butane. In fact, we should be able to compare these numbers to measure the energy transfer in [link] . Hess’ Law once again comes in handy as is illustrated in [link] . Now we can use an alternative pathway leading from reactants to products in two steps, first from reactants to the elements and then from the elements to the products. This means that we can write that the energy of [link] as
What about the other reactant in [link] , H 2 ? Why don’t we include the enthalpy of H 2 in the calculation? [link] doesn’t list an entry for the enthalpy of formation for H 2 . This is because the formation reaction for H 2 would simply be H 2 →H 2 , in other words, nothing needs to happen to form H 2 from elemental H 2 so there is no reaction and no enthalpy of formation for H 2 .
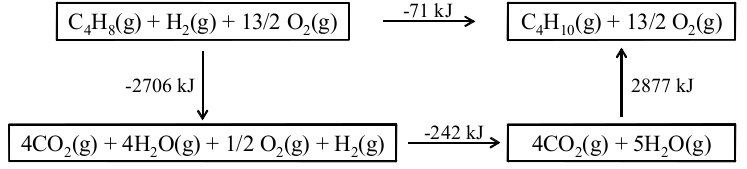
Our approach for finding ∆H of [link] is perfectly general. We can see that the heat of any reaction can be calculated from
For this reason, extensive tables of ΔHfº have been compiled and published. This allows us to calculate with complete confidence the heat of reaction for any reaction of interest, even including hypothetical reactions which may be difficult to perform or impossibly slow to react.
[link] is also a great way to interpret the energies of reactions. For example, if we observe an exothermic reaction, in other words if ∆H<0, we can say with confidence from [link] that ∆H f (reactants) is a larger number than ∆H f (products), that is, the reactants are higher in energy than the products.
Prior to this concept study and the previous one, we have discussed energy in the context of chemical bonding. We know that a chemical bond forms when the energy of the bonded atoms is lower than the energy of the separated atoms. The amount of energy required to break the bond and separate the atoms is called the bond energy. Since chemical reactions are all about breaking and making bonds, the energies of chemical reactions must be related to bond energies. Let’s make some new observations which allow us to develop this relationship.
Let’s observe two simple examples. First, the reaction
is observed to be endothermic with a heat of reaction of 70 kJ/mol. What happens in this reaction seems pretty simple. The reaction could be viewed as consisting entirely of the breaking of the H 2 bond followed by the formation of the HBr bond. Using Hess’ Law and following the process which first breaks the H 2 bond and then forms the HBr bond, we must input energy equal to the bond energy of H 2 (436 kJ/mol), and in forming the HBr bond we recover output energy equal to the bond energy of HBr (366 kJ/mol). The enthalpy of [link] must be equal to difference in these bond energies, 70 kJ/mol.
Note that it does not matter whether the reaction actually occurs by first breaking a bond and then forming a new one. In fact, it probably does not happen this way. The two actions, bond breaking and bond formation, actually happen simultaneously as the H atom moves smoothly from the other H atom to the Br atom. But, by Hess’ Law, we can follow the pathway which first breaks the bond and then forms the new bond and still get the energy of the reaction correct.

Notification Switch
Would you like to follow the 'Concept development studies in chemistry 2012' conversation and receive update notifications?