| << Chapter < Page | Chapter >> Page > |
The kinetic theory of gases was developed by Daniel Bernoulli (1700–1782), who is best known in physics for his work on fluid flow (hydrodynamics). Bernoulli’s work predates the atomistic view of matter established by Dalton.
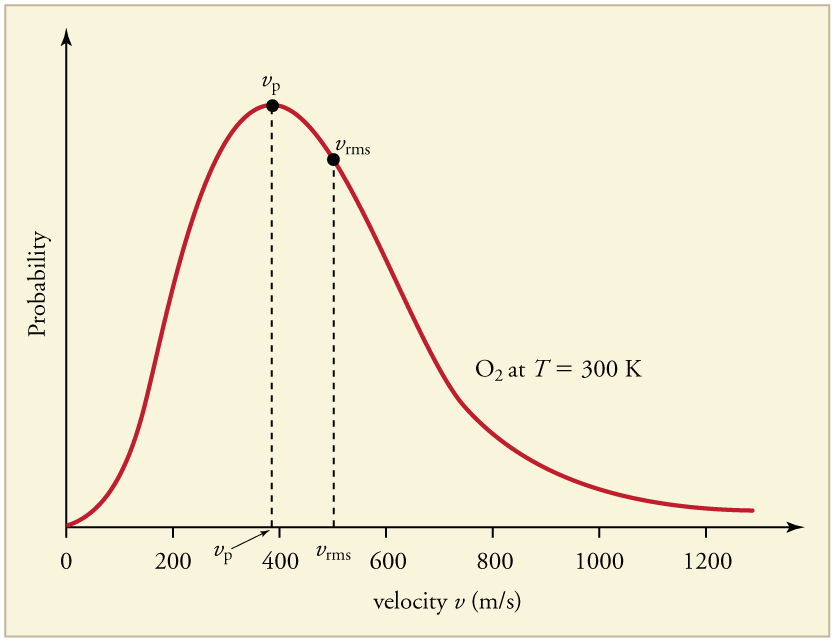
The motion of molecules in a gas is random in magnitude and direction for individual molecules, but a gas of many molecules has a predictable distribution of molecular speeds. This distribution is called the Maxwell-Boltzmann distribution , after its originators, who calculated it based on kinetic theory, and has since been confirmed experimentally. (See [link] .) The distribution has a long tail, because a few molecules may go several times the rms speed. The most probable speed is less than the rms speed . [link] shows that the curve is shifted to higher speeds at higher temperatures, with a broader range of speeds.
The distribution of thermal speeds depends strongly on temperature. As temperature increases, the speeds are shifted to higher values and the distribution is broadened.
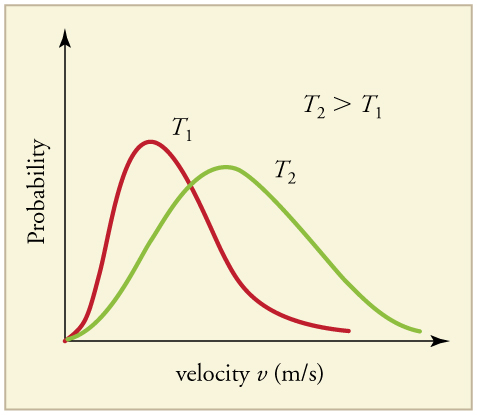
What is the implication of the change in distribution with temperature shown in [link] for humans? All other things being equal, if a person has a fever, he or she is likely to lose more water molecules, particularly from linings along moist cavities such as the lungs and mouth, creating a dry sensation in the mouth.
In order to escape Earth’s gravity, an object near the top of the atmosphere (at an altitude of 100 km) must travel away from Earth at 11.1 km/s. This speed is called the escape velocity . At what temperature would helium atoms have an rms speed equal to the escape velocity?
Strategy
Identify the knowns and unknowns and determine which equations to use to solve the problem.
Solution
1. Identify the knowns: is the escape velocity, 11.1 km/s.
2. Identify the unknowns: We need to solve for temperature, . We also need to solve for the mass of the helium atom.
3. Determine which equations are needed.
or
to yield
4. Plug the known values into the equations and solve for the unknowns.
Discussion
This temperature is much higher than atmospheric temperature, which is approximately 250 K or at high altitude. Very few helium atoms are left in the atmosphere, but there were many when the atmosphere was formed. The reason for the loss of helium atoms is that there are a small number of helium atoms with speeds higher than Earth’s escape velocity even at normal temperatures. The speed of a helium atom changes from one instant to the next, so that at any instant, there is a small, but nonzero chance that the speed is greater than the escape speed and the molecule escapes from Earth’s gravitational pull. Heavier molecules, such as oxygen, nitrogen, and water (very little of which reach a very high altitude), have smaller rms speeds, and so it is much less likely that any of them will have speeds greater than the escape velocity. In fact, so few have speeds above the escape velocity that billions of years are required to lose significant amounts of the atmosphere. [link] shows the impact of a lack of an atmosphere on the Moon. Because the gravitational pull of the Moon is much weaker, it has lost almost its entire atmosphere. The comparison between Earth and the Moon is discussed in this chapter’s Problems and Exercises.

Notification Switch
Would you like to follow the 'College physics' conversation and receive update notifications?