| << Chapter < Page | Chapter >> Page > |
| Term | Definition |
|---|---|
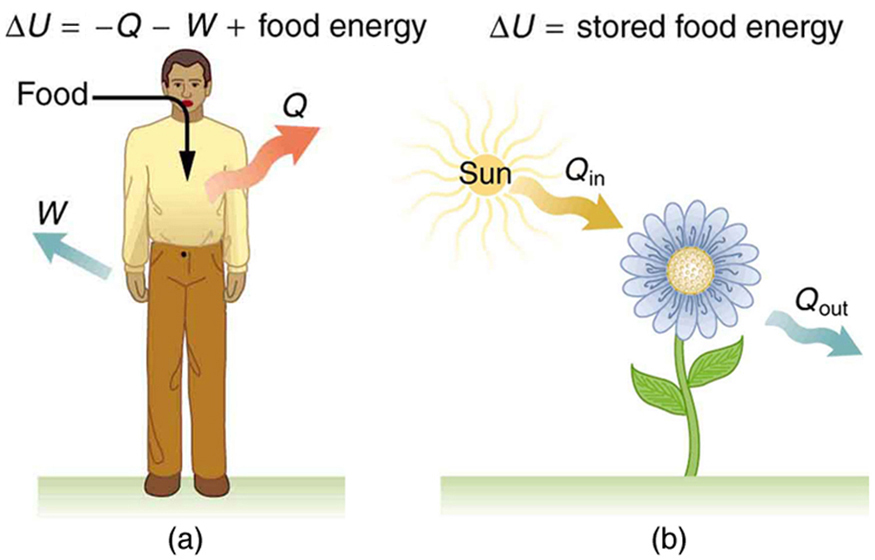
| Internal energy—the sum of the kinetic and potential energies of a system's atoms and molecules. Can be divided into many subcategories, such as thermal and chemical energy. Depends only on the state of a system (such as its , , and ), not on how the energy entered the system. Change in internal energy is path independent. | |
| Heat—energy transferred because of a temperature difference. Characterized by random molecular motion. Highly dependent on path. entering a system is positive. | |
| Work—energy transferred by a force moving through a distance. An organized, orderly process. Path dependent. done by a system (either against an external force or to increase the volume of the system) is positive. |
A cylinder is divided in half by a movable disk in the middle. Each half is filled with an equal number of gas molecules, but one half is at a higher temperature than the other. Which choice best describes what happens next?
(d)
Imagine a solid material at the molecular level as consisting of a bunch of billiard balls connected to each other by springs (this is actually a surprisingly useful approximation). If we have two blocks of the same material, but in one the billiard balls are shaking back and forth on their springs a great deal, and in the other they are barely moving, which block is at the higher temperature? Using what you know about conservation of momentum in collisions, describe which block will transfer energy to the other, and justify your answer.
A system has 300 J of work done on it, and has a heat transfer of -320 J. Compared to prior to these processes, the internal energy is:
(a)
Find a snack or drink item in the classroom, or at your next meal. Find the total Calories (kilocalories) in the item, and calculate how long it would take exercising at 150 W (moderately, climbing stairs) at 20% efficiency to burn off this energy.
A potato cannon has the fuel combusted, generating a lot of heat and pressure, which launch a potato. The combustion process _____ the internal energy, while launching the potato _____ the internal energy of the potato cannon.
(b)
Describe what happens to the system inside of a refrigerator or freezer in terms of heat transfer, work, and conservation of energy. Confine yourself to time periods in which the door is closed.

Notification Switch
Would you like to follow the 'College physics for ap® courses' conversation and receive update notifications?