| << Chapter < Page | Chapter >> Page > |
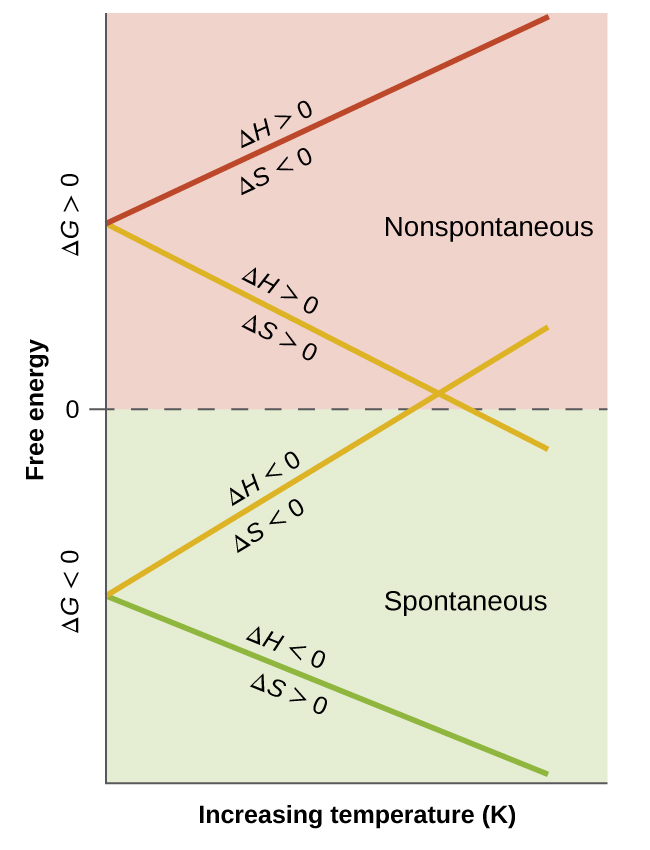
Such a plot is shown in [link] . A process whose enthalpy and entropy changes are of the same arithmetic sign will exhibit a temperature-dependent spontaneity as depicted by the two yellow lines in the plot. Each line crosses from one spontaneity domain (positive or negative Δ G ) to the other at a temperature that is characteristic of the process in question. This temperature is represented by the x -intercept of the line, that is, the value of T for which Δ G is zero:
And so, saying a process is spontaneous at “high” or “low” temperatures means the temperature is above or below, respectively, that temperature at which Δ G for the process is zero. As noted earlier, this condition describes a system at equilibrium.
When this process is at equilibrium, Δ G = 0, so the following is true:
Using the standard thermodynamic data from Appendix G ,
The accepted value for water’s normal boiling point is 373.2 K (100.0 °C), and so this calculation is in reasonable agreement. Note that the values for enthalpy and entropy changes data used were derived from standard data at 298 K ( Appendix G ). If desired, you could obtain more accurate results by using enthalpy and entropy changes determined at (or at least closer to) the actual boiling point.
313 K (accepted value 319 K)
The free energy change for a process may be viewed as a measure of its driving force. A negative value for Δ G represents a finite driving force for the process in the forward direction, while a positive value represents a driving force for the process in the reverse direction. When Δ G is zero, the forward and reverse driving forces are equal, and so the process occurs in both directions at the same rate (the system is at equilibrium).
In the chapter on equilibrium the reaction quotient , Q , was introduced as a convenient measure of the status of an equilibrium system. Recall that Q is the numerical value of the mass action expression for the system, and that you may use its value to identify the direction in which a reaction will proceed in order to achieve equilibrium. When Q is lesser than the equilibrium constant, K , the reaction will proceed in the forward direction until equilibrium is reached and Q = K . Conversely, if Q < K , the process will proceed in the reverse direction until equilibrium is achieved.

Notification Switch
Would you like to follow the 'Chemistry' conversation and receive update notifications?