| << Chapter < Page | Chapter >> Page > |
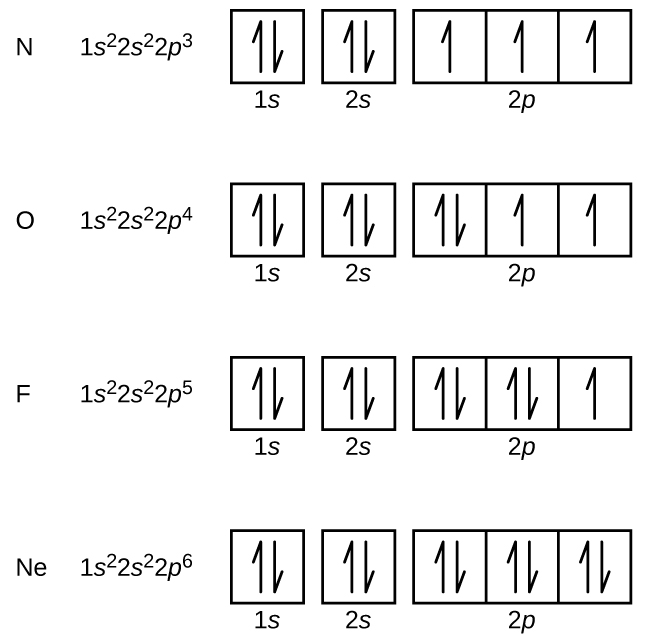
The alkali metal sodium (atomic number 11) has one more electron than the neon atom. This electron must go into the lowest-energy subshell available, the 3 s orbital, giving a 1 s 2 2 s 2 2 p 6 3 s 1 configuration. The electrons occupying the outermost shell orbital(s) (highest value of n ) are called valence electrons , and those occupying the inner shell orbitals are called core electrons ( [link] ). Since the core electron shells correspond to noble gas electron configurations, we can abbreviate electron configurations by writing the noble gas that matches the core electron configuration, along with the valence electrons in a condensed format. For our sodium example, the symbol [Ne] represents core electrons, (1 s 2 2 s 2 2 p 6 ) and our abbreviated or condensed configuration is [Ne]3 s 1 .
![This figure includes the element symbol N a, followed by the electron configuration for the element. The first part of the electron configuration, 1 s superscript 2 2 s superscript 2 2 p superscript 6, is shaded in purple and is labeled, “core electrons.” The last portion, 3 s superscript 1, is shaded orange and is labeled, “valence electron.” To the right of this configuration is the word “Abbreviation” followed by [ N e ] 3 s superscript 1.](/ocw/mirror/col11760/m51041/CNX_Chem_06_04_Valence.jpg)
Similarly, the abbreviated configuration of lithium can be represented as [He]2 s 1 , where [He] represents the configuration of the helium atom, which is identical to that of the filled inner shell of lithium. Writing the configurations in this way emphasizes the similarity of the configurations of lithium and sodium. Both atoms, which are in the alkali metal family, have only one electron in a valence s subshell outside a filled set of inner shells.
The alkaline earth metal magnesium (atomic number 12), with its 12 electrons in a [Ne]3 s 2 configuration, is analogous to its family member beryllium, [He]2 s 2 . Both atoms have a filled s subshell outside their filled inner shells. Aluminum (atomic number 13), with 13 electrons and the electron configuration [Ne]3 s 2 3 p 1 , is analogous to its family member boron, [He]2 s 2 2 p 1 .
The electron configurations of silicon (14 electrons), phosphorus (15 electrons), sulfur (16 electrons), chlorine (17 electrons), and argon (18 electrons) are analogous in the electron configurations of their outer shells to their corresponding family members carbon, nitrogen, oxygen, fluorine, and neon, respectively, except that the principal quantum number of the outer shell of the heavier elements has increased by one to n = 3. [link] shows the lowest energy, or ground-state, electron configuration for these elements as well as that for atoms of each of the known elements.
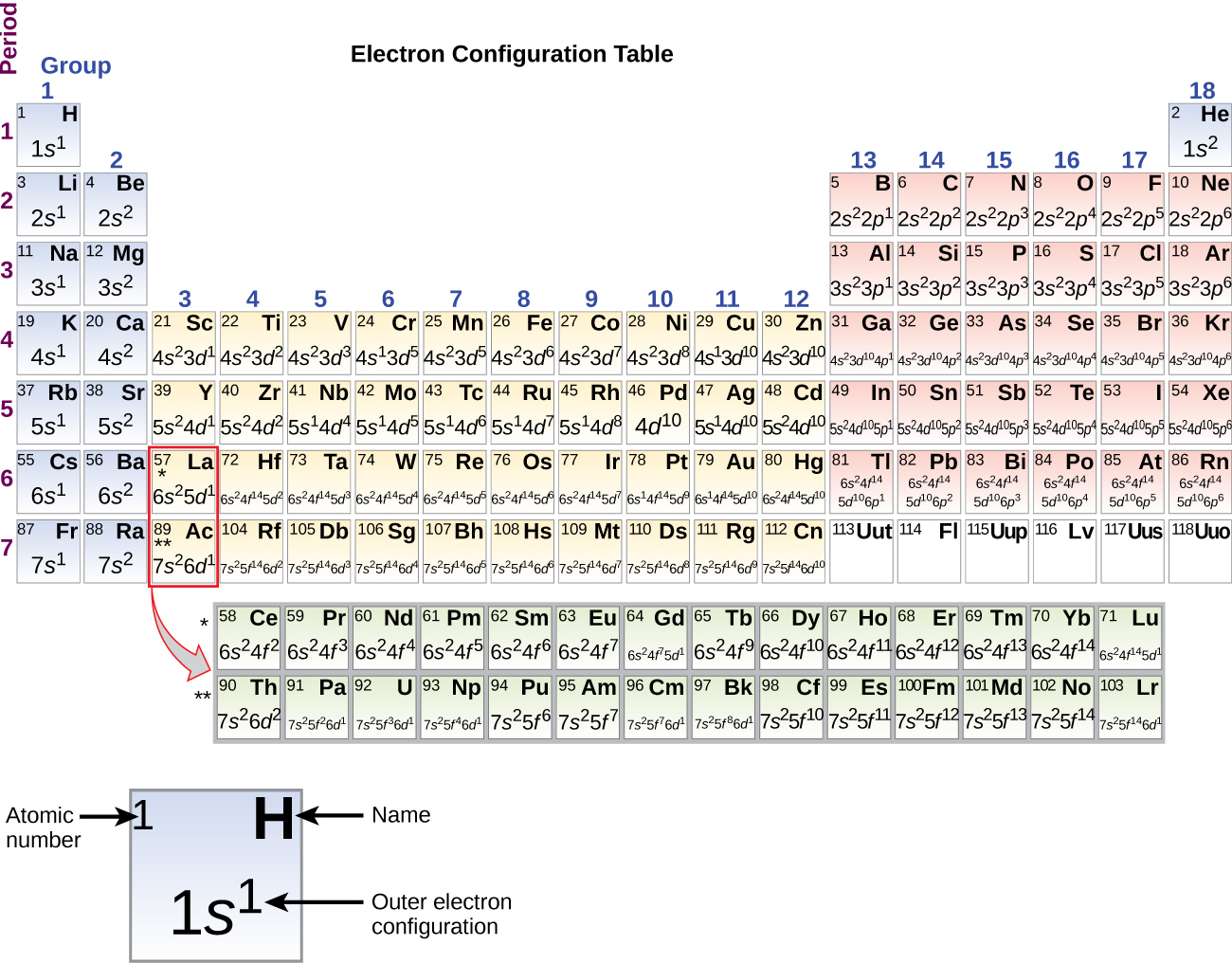
When we come to the next element in the periodic table, the alkali metal potassium (atomic number 19), we might expect that we would begin to add electrons to the 3 d subshell. However, all available chemical and physical evidence indicates that potassium is like lithium and sodium, and that the next electron is not added to the 3 d level but is, instead, added to the 4 s level ( [link] ). As discussed previously, the 3 d orbital with no radial nodes is higher in energy because it is less penetrating and more shielded from the nucleus than the 4 s , which has three radial nodes. Thus, potassium has an electron configuration of [Ar]4 s 1 . Hence, potassium corresponds to Li and Na in its valence shell configuration. The next electron is added to complete the 4 s subshell and calcium has an electron configuration of [Ar]4 s 2 . This gives calcium an outer-shell electron configuration corresponding to that of beryllium and magnesium.

Notification Switch
Would you like to follow the 'Chemistry' conversation and receive update notifications?