| << Chapter < Page | Chapter >> Page > |

Watch a brief
video showing the test firing of a small-scale, prototype, hybrid rocket engine planned for use in the new Space Launch System being developed by NASA. The first engines firing at
3 s (green flame) use a liquid fuel/oxidant mixture, and the second, more powerful engines firing at 4 s (yellow flame) use a solid mixture.
Single-displacement (replacement) reactions are redox reactions in which an ion in solution is displaced (or replaced) via the oxidation of a metallic element. One common example of this type of reaction is the acid oxidation of certain metals:
Metallic elements may also be oxidized by solutions of other metal salts; for example:
This reaction may be observed by placing copper wire in a solution containing a dissolved silver salt. Silver ions in solution are reduced to elemental silver at the surface of the copper wire, and the resulting Cu 2+ ions dissolve in the solution to yield a characteristic blue color ( [link] ).
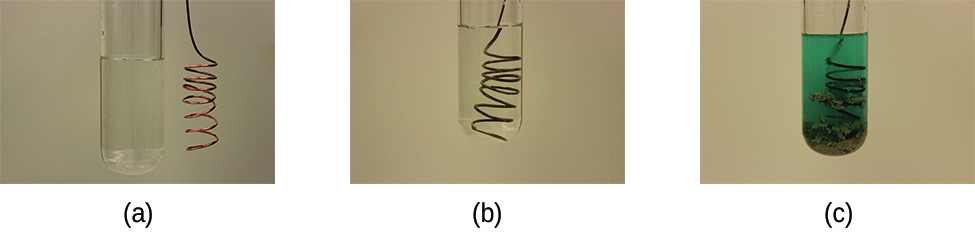
(a)
(b)
(c)
(d)
(e)
(a) This is not a redox reaction, since oxidation numbers remain unchanged for all elements.
(b) This is a redox reaction. Gallium is oxidized, its oxidation number increasing from 0 in Ga( l ) to +3 in GaBr 3 ( s ). The reducing agent is Ga( l ). Bromine is reduced, its oxidation number decreasing from 0 in Br 2 ( l ) to −1 in GaBr 3 ( s ). The oxidizing agent is Br 2 ( l ).
(c) This is a redox reaction. It is a particularly interesting process, as it involves the same element, oxygen, undergoing both oxidation and reduction (a so-called disproportionation reaction) . Oxygen is oxidized, its oxidation number increasing from −1 in H 2 O 2 ( aq ) to 0 in O 2 ( g ). Oxygen is also reduced, its oxidation number decreasing from −1 in H 2 O 2 ( aq ) to −2 in H 2 O( l ). For disproportionation reactions, the same substance functions as an oxidant and a reductant.
(d) This is not a redox reaction, since oxidation numbers remain unchanged for all elements.
(e) This is a redox reaction (combustion). Carbon is oxidized, its oxidation number increasing from −2 in C 2 H 4 ( g ) to +4 in CO 2 ( g ). The reducing agent (fuel) is C 2 H 4 ( g ). Oxygen is reduced, its oxidation number decreasing from 0 in O 2 ( g ) to −2 in H 2 O( l ). The oxidizing agent is O 2 ( g ).
Is this a redox reaction? If so, provide a more specific name for the reaction if appropriate, and identify the oxidant and reductant.
Yes, a single-replacement reaction. Sn( s ) is the reductant, HCl( g ) is the oxidant.

Notification Switch
Would you like to follow the 'Chemistry' conversation and receive update notifications?